Corpus Vitrearum Medii Aevi
Medieval Stained Glass in Great Britain
Your trail:
- Conservation
- Conservation: Materials and Methods
Conservation: Materials and Methods
(Elisabeth Jägers, Hannelore Römich and Carola Müller-Weinitsche)
1. Conservation Materials for Historical Windows
A wide variety of natural and synthetic materials is used in the conservation and restoration of stained glass, variously for repair of fractures and edge-bonding, fixing loose paint layers, or as a protective coating for vulnerable areas. These substances, generically referred to as bonding agents, are applied as a solution, emulsion or glaze, and dry out to form a hard and adhesive film. They are mostly polymers, in other words macro-molecules with various chemical components.
All such substances, regardless of their various components and functions, should conform to the following requirements. They should ideally be as malleable as possible at room temperature in order to avoid unnecessary thermal stress to the glass, and they should be able to be used in the workshop without recourse to sophisticated equipment or expenditure on technology. Most importantly, in order to achieve success when edge bonding or protecting a surface, they should have and maintain firm adhesion to the surface of the glass, with the minimum of visual disturbance to the latter's appearance. In addition to this, there should be high expectations of the ageing process of conservation materials: they should be chemically stable, retaining their composition and characteristics during the ageing process, so that they are capable of being removed at any time: in other words, their application should be reversible.
Within the conservation profession, the principle of reversibility has been defined and assessed in very different ways (Petzet 1992). In general, bonding agents are considered reversible when they remain removable with organic solvents after a considerable period of time. In practice it is recognised that total removal of such substances may often prove impossible due to the porosity of ancient glass surfaces, whether painted, worn or severely corroded. Nevertheless, the principle that bonding agents should not age should be adhered to as closely as possible, in order to reduce the chances of uncontrolled and potentially dangerous material changes to the glass, such as the glass becoming more yellow, more brittle, or more stressed.
Beyond these general principles, substances used in glass conservation should also possess particular qualities depending on their intended use. For example, protective substances should prevent further corrosion by providing an effective insulating external layer against water and moisture. Substances used to conserve paint layers should aim to bind the loose paint particles to the glass by penetrating their porous matrix. In this case the development of an external film is undesirable, as this creates a risk that the binding agent may with age peel away from the glass, taking the paint with it. Adhesives on the other hand should possess, besides of course suitable strength, should be malleable and removable, so that they may be of use even in complex cases of edge-bonding.
In modern conservation practice a range of synthetic originally developed for industrial applications bonding agents is used. These bonding agents have been modified for use on ancient corroded glass, for example by choosing different solvents and thinners, or by varying the strength and method of application. However, it is rare that such modified materials are entirely suitable for their chosen use, and their application often involves a degree of compromise. This is another good reason why the use of conservation materials should be limited to the absolutely necessary, both in terms of the area affected and the amount used. Localized use is always preferable to application over a large area.
The risks involved in the use of such substances often only become clear after several years, when the effects of natural ageing and environmental factors can be assessed. Familiarity with the history of restoration shows again and again that materials considered to be the ideal solution at the time can sometimes have dramatic and damaging long-term effects. Restorations undertaken recently can be particularly problematic, as the process of removing the substance, whether used as an adhesive, paint consolidator or in the plating process, can be risky and technically complex.
Natural ageing through oxidization and chemical interaction can alter the characteristics of polymers; this can lead to opacity, cracking or to loss of adhesion due to shrinkage. As well as the risk of changes in the conservation materials over time caused by environmental factors such as light, oxygen and pollution, there is also the possibility that the various conservation substances (e.g., the different agents used for repair, protective layers, paint consolidation, or cold retouching) may react with one another.
The foci of the research described in the following section were the composition and characteristics of materials used in glass conservation in the recent past, and their long-term effects on the condition of the stained glass on which it was used (its damage-causing potential). One of the main objectives was to develop methods and materials suitable for reversing a conservation process; another was to examine the materials presently in use and new substances being developed in order to establish their effectiveness and durability.
As well as examining samples of bonding agents in situ, a wide range of scientific techniques is available for laboratory analysis in order to establish their composition and characteristics. It is important to ensure that such laboratory programmes are based on practical experience, to create the most authentic test conditions possible. For example, one can only test the effectiveness of protective layers by applying them to glass samples that are sensitive and also corroded and dirty. By contrast, with substances intended for use fixing loose paint layers, the ability to infiltrate porous layers is more important than adhesive strength.
Adequate and relevant conservation criteria should guide the analysis of the results. In order to avoid any false interpretation, close co-operation between scientists and conservators is necessary, not just to formulate the questions being asked and design the test programmes, but also to test the results and their practical application. Material testing in laboratories usually relies on artificially produced weathering conditions, and although it is possible to analyze the ageing process of the polymers affected in a relatively short space of time, it is impossible to extrapolate a realistic time scale for these effects in natural conditions. While laboratory experiments may therefore establish preliminary information about certain matters (for example, which of a series of conservation materials is the most durable under certain atmospheric-pollution conditions), it is not possible to evaluate the exact lifespan of a particular substance.
Nevertheless, such tests can provide important information about the suitability of such substances under real conditions. Under no circumstances should materials developed for industrial use be tried in conservation practice without such testing. The most important prerequisite for a conservation project to succeed is therefore a case study specific to that project: only on the basis of this can the correct materials and application method be established, never forgetting the potential risks to the artwork.
2. Research on Restoration Materials and Methods Used in Earlier Conservation Projects
2.1. Introduction
The history of conservation agents - the use of organic bonding agents in the conservation of stained glass -is relatively short. Up to the late twentieth century, traditional mechanical methods were used to repair ancient glass: corroded glass was replaced, cracks mended with repair leads, damaged areas of paintwork repainted with stained glass paint and refired - that is, if complete replacement copy was not contemplated from the outset.
The trend towards using new materials for conservation and restoration that had not previously featured in the stained glass tradition began in the early twentieth century, in most cases closely following the development of synthetic resins as bonding agents and conservation agents; in many respects, these resins offered considerable advantages over traditional materials. The result was a shift from the craft-based conservation or renovation of stained glass towards treatments that were more or less substance-dependent: the new synthetic agents allowed fractures to be edge-bonded, loose paint layers to be secured, and glass to be conserved by coating it over a large area with a bonding agent or by plating individual segments of glass. These materials were simple to apply and due to their particular qualities offered the potential for new, previously unimagined applications. The history of modern stained glass restoration is therefore closely bound up with the development and diffusion of synthetic materials. The use of natural materials common in other conservation disciplines, such as lime, shellac or wax, was relatively rare.
A comprehensive overview of the use of conservation materials over this period can be found in Roy Newton's 'Bibliography of the Conservation of Stained Glass' (Newton and Davison 1989). The fundamental problems involved in the use of synthesized organic substances, particularly for stabilising paint layers, is dealt with in a number of publications by Eva Frodl-Kraft (Frodl-Kraft 1963; Frodl-Kraft 1970).
In general, the use of new materials for the conservation of historical stained glass should be considered as a positive development. It is no longer necessary to replace damaged glass, instead these materials can be uses to preserve it. However, well intentioned conservation procedures have not always been successful in the past. Many of the new substances failed over the long term to deliver the results promised by their producers: they have changed their characteristics over time, becoming darker or brittle, often endangering the stability of the glass for the future.
One should not forget that these synthetic substances were not developed for conservation purposes, but rather as industrial adhesives or as varnishes for consumer goods. As such they were designed for quite different purposes from those for which they were then used in glass restoration and conservation. Then as now, one will find nothing in the instruction manuals for these materials concerning their long-term qualities and effects and their compatibility with ancient and weakened glass.
2.2. Objectives of the Research
The objective of the research presented below was the analysis and assessment of materials and methods used in the past, in so far as they can still be detected on historical stained glass today. This research on glass that had been conserved and restored previously, and which had subsequently been exposed to the effects of time and the elements, opened up the possibilities for expanding our knowledge in the following areas.
- By observing real samples and studying naturally aged bonding-agent systems phenomenologically and analytically, it would be possible to gain new insights into the long-term effects of conservation materials and methods beyond the knowledge that can be obtained from artificially aged samples and laboratory simulations. In order for this to be successful, sufficient documentary evidence of the original conservation or restoration work had to be available.
- Through this research, it would be possible to determine whether and how historical conservation substances had reacted with the old glass and corrosion products. This research would examine physical and chemical changes, as well as secondary reactions (e.g., the effects of micro-organisms).
- By using the knowledge gained about materials and their characteristics, it would be possible to develop appropriate procedures for reversing a restoration process, removing ageing conservation materials safely in those cases in which the research had shown such a process to be necessary.
2.3. Sample Materials and Research Methods
Samples were made available for analysis by the stained glass studios of Cologne and Erfurt cathedrals, and by the Oidtmann, Linnich and van Treeck workshops in Munich. All of the glass samples had already been removed for conservation after damage or changes in the material. The original goal of the analysis of these samples was therefore to provide support in the conservation process, rather than to achieve a comprehensive survey of historical conservation materials. The new information gained from these individual conservation projects did not therefore at first appear to be of particular value, until all the results from these projects were analyzed and compared, facilitating a historical overview of conservation materials.
Establishing the identity and characteristics of organic compounds, particularly those of synthetic or organic macromolecules, naturally proved to be far more difficult than that of inorganic substances. In most cases, a range of complementary techniques had to be tried. 1 In the research presented here, the techniques included the use of microscopy with top light, polarized light, and ultra-violet light, often using thin sections of the samples; micro-chemical analysis; and infra-red spectroscopy. The latter proved to be the most useful technique, as it is flexible and well suited to identify both inorganic and inorganic substances.
Only tiny samples were available for analysis from figurative glass, because each time a sample is created, the substance of the glass is destroyed. The taking of samples must therefore be reduced to an absolute minimum, and analysis should be carried out at a microscopic level in order to avoid such destruction as far as possible. In other words, one must attempt to provide the maximum number of answers - to often very different questions - from a minimum of material. 2 Under these circumstances, it is extremely important to make a precise record of the sample, the area from which the sample was taken, its composition and condition; such documentation has an influence on the conclusions and the validity of the research itself, as well as for its application in conservation practice.
2.4. Overview of Methods for Stained Glass Restoration and Conservation
The restoration and conservation of stained glass requires a phased approach, whereby a wide range of materials will be necessary, depending on the nature of the task at hand. A bonding agent used for fracture repair and edge-bonding should obviously have strong and long-lasting adhesive qualities, and should also dry quickly and be easy to apply. On the other hand, a bonding agent used for fixing loose paintwork on stained glass should form a film, be capable of penetrating the loose glass structure beneath and forming a firm connection with the base layer, drying to a matt finish; adhesive strength is less important. In addition to specific optical and adhesive qualities, a film of bonding agent applied to protect the surface of the glass and provide protection against corrosion requires durability and an ability to resist the elements.
Numerous studies on stained glass conserved in the last few decades have shown that the earlier conservators tended not to choose their materials on the basis of the above criteria. Rather, industrial adhesives and bonding agents were used without being modified or optimized. Fracture repair and edge-bonding were carried out using glass glues, generally dual-component glues with an epoxy or polyester base (see table 1). These glues increasingly replaced the traditional molten lead or solder repairs, though these were still used sometimes. Other synthetic adhesives used in the repair of breaks, such as silicon and acrylics, have only played a relatively minor role, even in recent years.
| Binding agent | Type of material or product name | Usage | Characteristics of film when aged |
|---|---|---|---|
| polyvinyl acetate (PVAc | Mowilith 35/73 (UHU all-purpose glue or other glues common in the trade) | a. plating | a. thick, blistered in appearance, though with no change in the colouration |
| b. application of a coating or crack repair by application over an area | b. browned and flaky, lacking adhesion to the glass | ||
| acrylic resin | Paraloid B72 ethylmethacrylate / methyl-acrylate co-polymer (EMA/MA, made by Röhm & Haas) | a. stabilization of layers | a. unchanged |
| b. application in coats with fine surface cracking | b. thick, blistered, flaking off in places | ||
| c. crack repair | c. unchanged, scarcely elastic | ||
| Plexidon M449 polymethylmethacrylate (PMMA, made by Röhm) | used for plating in the Jacobi method | unchanged, and very soft on account of the softeners | |
| polyethylenimin | Hostacoll C (made by Hoechst) | crack repair in combination with Jacobi-method plating | browned, extremely brittle, and completely broken down |
| epoxy resins | |||
| 1. types of Araldite | AY101/HY951 diglycidyl ether of Bisphenol A (DGEBA) + triethyltetramine (TETA | plating | browned, brittle |
| AY103/HY951 DGEBA + TETA containing softeners | layer stabilization, coating of surfaces in 1970 | very browned, seemingly not brittle when applied thinly | |
| AY103/HY956 DGEBA + | crack repair | browned | |
| TETA/TEPA (tetraethylenepentamin) containing softeners | layer stabilization, coating of surfaces in 1980 | brittle, browned | |
| XW396/XW397 DGEBA + BGA (butyl glycidyl ether) | crack repair | slightly browned? | |
| 2. type of Disbon | aliphatic epoxy resin | crack repair and coating of large areas | browned, brittle, flaky, lacking adhesion to the glass |
| Polyester | unsaturated polyester combined with Styrol (made by Akemi) | crack repair and coating of large areas | browned, brittle, flaky, lacking adhesion to the glass |
| Silicon resin | BS31/TU2 (made by Wacker) | layer stabilization and coating of large areas | slightly cloudy film, high tension |
| self-adhesive foils | a. latex resin adhesive | emergency repairs, break repairs | browned, brittle |
| b. acrylic resin or PVAc-acrylic co-polymer | emergency repairs, application of adhesive over large areas | The softeners leaked out! |
The range of bonding agents used for the conservation of paint layers was of a different order. It is clear that there was a great deal of experimentation with a wide variety of methods and materials. In many cases, large areas of glass were treated with bonding agents, usually on the inner glass surface, in order to fix or stabilize loose paint layers. Targeted application limited to damaged areas was rare, probably for technical reasons. This of course means that the paint layers were necessarily affected by unexpected changes in these bonding agents over time, and indeed by any attempts to remove them. A wide variety of substances was also used to create a film to protect external glass surfaces from the weather, and here too large areas of glass were treated. The most major changes, which often led to damage to the stained glass, were observed with these substances, as they were directly exposed to light and weather. For these reasons, the analysis of these materials and their long-term qualities was a priority in this study.
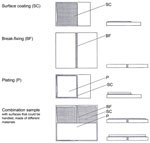
In addition to the various techniques adopted for applying a protective layer to glass using organic and inorganic bonding agents, two plating procedures are of particular significance for the conservation history of stained glass: the first (Jacobi's method) using acrylic resin adhesive (Brinkmann and Decker 1995), the second using epoxy resins (Jägers and Brinkmann 1999). In both methods, damaged (i.e., corroded and often cracked) glass had a layer of suitable pre-cut plate glass stuck to it with a layer of adhesive (as with safety-glass technology), in order to protect the original from further damage from the weather or other corroding factors. The two methods are immediately comparable, though there are important differences in the adhesives used: one is a thermoplastic acrylic adhesive (which is in principle soluble and therefore could be dissolved at any point), and the other is an epoxy resin (which, after hardening in organic solution, is completely insoluble in practice).
Both plating methods were controversial from the start, because it was not just the visual effect of the glass that was affected for the worse. For technical reasons, Jacobi's plating technique involving acrylic resin required the original leading to be replaced; with the plating technique involving epoxy resin, the adhesive quickly showed signs of yellowing and becoming brittle. Part of the research was therefore directed towards analyzing these methods and the characteristics of the adhesives used, in order to develop, among other things, methods and materials for their removal.
In what follows the materials most commonly used in stained glass conservation (as established by the research) will be discussed, focussing especially on the substances themselves, their material classification, and qualities (in particular their ageing profile), analyzing the consequences of this for the survival of the stained glass.
2.5. Early Examples of the Use of Both Natural and Synthetic Conservation Materials
The residues of various substances - generally oil-based - are frequently found on the surfaces of glass, yielding information on the use of natural organic bonding agents. However, with such oil-based deposits, it is not always easy to differentiate between a conservation agent, overpainting, a pigmented varnish (possibly a resin-oil- or asphalt-based lacquer), or perhaps just the residues of a linseed oil putty used for cementing.
Generally, the relevant sources or restoration documentation will contain information on the use of waxes, oils and resins used in painting or applying a protective layer, but proving the existence of these materials is very hard to achieve, because organic bonding agents over time are extensively broken down and lost through natural ageing processes and especially the action of micro-organisms. Indeed, evidence for the action of micro-organisms is often found on stained glass, and this can consist of the growth of hyphae, or traces of oxalates (oxalic acid salts), typical by-products of the metabolic processes of living organisms. The presence of micro-organisms, whether bacteria or fungi, can be taken as proof of the application of organic bonding agents to the glass surface.
It is generally easier to identify wax-based substances. Wax has a special status within the palette of materials used in glass conservation, as it is considered a chemically stable and extremely durable bonding agent, still in common use for the conservation of paint layers on sculpture or paintings on panels or canvas. It is still occasionally used in stained glass restoration, for example recently on the extremely fragile and endangered layers of paint on the choir windows at Erfurt Cathedral. Here the loose layers were fixed with a warmed mixture of beeswax and Carnauba wax (Drachenberg and Müller 1980).
Inorganic bonding agents were also used in glass conservation in the early twentieth century. One particularly striking technique involved the application of molten glass directly onto corroded glass surfaces, an idea that appeared logical at the time. This idea was translated into practice by Zettler, whose technique involved cleaning the affected area thoroughly and then applying glass powder that was then melted onto the surface. It was used on the windows of the churches of St Lorenz and St Sebald in Nuremberg, where the disadvantages of this technique can clearly be seen (Frenzel 1969; Newton and Davison 1989). The glass has darkened and become opaque, and the surface appears encrusted because corrosion and dirt was unavoidably mixed with the applied glass powder ( 1). This fused with the original surface and is now impossible to remove with any known solvent, making restoration of this glass impossible.
At the beginning of the twentieth century, protective layers of water glass, otherwise known as sodium- or potassium water glass, were applied on occasion. This material was produced by heating ground quartz with soda (Na2CO3) or potash (K2CO3). It had been rediscovered by Nepomuk von Fuchs in the mid-nineteenth century and put to a variety of uses, for example for fire-proofing timber, the producing ultramarine, developing a special painting technique (painting with mineral colours), conserving plaster and stone, etc. (Schiessl 1985). It was a short step to using water glass, which dried to a glass-like layer, for the conservation of stained glass; indeed, during our research examples of glass with a thin, greyish, transparent external layer were found, which due to its structure and weak adhesion to the glass surface could be interpreted as a film applied for conservation purposes. Analysis showed this to be silica gel. However, since dried water glass differs very little from the worn out surface of ancient glass - both are polysilicic acids - it is very difficult to confirm the presence of the former unambiguously.
The oldest example of restoration using synthetic organic bonding agents that we were able to identify was the use of cellulose nitrate, found in the choir windows at Erfurt Cathedral. The layers of cellulose lacquer applied by the Linnemann Glass Workshop from 1909 to 1911 on five of these windows (nIV-VIII) with the intention of preserving the paint pigments is unfortunately now either lost or infiltrated by layers of corrosion, which has caused the lacquer and the paint layers to which it is attached to flake off.
Cellulose nitrate was the earliest example of an artificially produced bonding agents being used in conservation. It had been used as an adhesive for glass and ceramics, for crazed enamel, and for timber and metal restoration (Selwitz 1988). Due to its poor durability, thermal and photochemical instability, and tendency to become more brittle over time (resulting, among other things, from the presence of softening agents), cellulose nitrate was soon replaced by other newly developed artificial resins.
2.6. Synthetic Bonding Agents as Conservation Materials
The majority of the glass samples analyzed dated to the last 20-30 years, the most recent period of the history of glass conservation. The bonding agents identified were without exception artificial resins, i.e., synthetic bonding agents. The range of artificial resin types identified varied from very soft, soluble resins based on polyvinyl acetates or their co-polymers (thermoplastics), to extremely hard, almost insoluble dual-component resins based on epoxy, polyester or silicon resins (duroplastics).
The big advantage of the duroplastic dual-component resins (reactive resins) over the thermoplastic polymer resins (polyvinyl acetates and acrylic resins) is their superior strength and superior ability to adhere to the base layer. Both qualities derive from their molecular structure. While setting, a duroplastic develops a three-dimensional macromolecular network that is insoluble and thermally neutral. A thermoplastic on the other hand consists of linear or branching single molecules that hold together through molecular mutual attraction. These are relatively weak in comparison to real chemical combinations, and can easily be broken down with heat or solvents, with the resin softening or dissolving. By contrast epoxy resin or polyester adhesives are very hard and resilient, but also insoluble.
Furthermore the polarized molecular structure of the duroplastic resins allows them to build a stronger connection to the equally polarized glass surfaces, which increases their adhesive strength in comparison with the relatively unpolarized polymerized resins. This can however be a danger if the strength of the glue or the adhesion of the protective film exceeds the innate strength of the matrix conserved in this way. This can lead to secondary cracking adjacent to glued areas, or detachment or exfoliation of the outer glass layers due to the stresses exerted by the binding agent. Such effects were noted on several occasions during the study. Furthermore, thermoplastics and duroplastics differ in their durability. The polarized molecular structure of the dual-component resins mentioned above renders them liable to oxidization through photochemical reactions. This can lead to yellowing or discolouration of the glass, and generally goes hand in hand with changes in the physical characteristics of the film, which often becomes fragile. The molecular structure of the thermoplastics means that they are less vulnerable to such changes.
Adhesive strips and foils are a special category within the synthetic conservation materials, and were found to have been used surprisingly often. They had not only been used, as expected, as a temporary protective measure, but also for the permanent repair of large areas of cracked or crazed glass, and even to stabilize paint layers.
An overview of the conservation materials identified during the study is shown in table 1. In the following section the qualities and components of the most important bonding agents identified are described, with emphasis on the ageing characteristics of these resins and their various negative effects on the stained glass, with suggestions for their removal or reversal (Davison 1984; Horie 1987).
Polyvinyl acetates
Polyvinyl acetates are among the most important and most commonly used polymerized resins. They are used in the form of homo- or co-polymers with varying molecular weights in soluble form, or as water-based dispersions. 3 Their most prominent qualities are the relatively low point at which they melt to form a glaze (c.26C°) 4 and the resultant physical characteristics of the film (ranging from soft to toughly elastic), as well as their high adhesive strength. They are thought to be durable resins that are not liable to darken. They are, however, sensitive to moisture and therefore vulnerable if used as an external protective layer. The polyvinyl-acetate resins identified during the study were all mini-molecular products that could be easily dissolved by solvents. The use of polyvinyl-acetate solutions could however not be conclusively established. Polyvinyl acetates were used, for example, as glue for plating or generously applied over large areas to consolidate paint contours. In these cases, the typically poor adhesive qualities associated with polymerized resins could be observed; the film had parted company at least partially from the glass surfaces, and was covered in fine cracks and flakes. However, since polyvinyl-acetate resins -or at least the micro-molecular products described above - do not lose their solubility over time, they can at least be removed relatively unproblematically with solvents. One should use solvents of medium polarity, such as ethanol, ethyl acetate, methyl ethyl keton or similar. Even the polyvinyl acetates used for plating can be removed this type of solvent.
Polyacrylates
The acrylate polymers (acrylic-acid esters) and methacrylate polymers (methacrylic-acid esters) and their co-polymers are similar to polyvinyl acetates, in that they are also soluble thermoplastic resins. They are considered particularly durable, and have been used for many years in the various branches of restoration and conservation, mostly as bonding agents for paint, lacquers and coatings. However, their adhesive qualities in comparison with the polyvinyl acetates are less advantageous. Their qualities vary greatly depending on their composition and molecular size, for example their solubility, the physical characteristics of film they form, and the temperature at which melt to form a glaze. As with the polyvinyl acetates, a distinction is made between micro-molecular acrylic resins that are soluble, and macro-molecular ones applied as dispersions.
Paraloid B72, developed by the firm Rohm & Haas, 5 has a special status within the acrylic resins. It is an ethyl methacrylate/methylacrylate co-polymer (in the proportion 70% / 30%) with a transformation point Tg of 40°C. This product has been widely used in conservation practice since the 1970s. It has been tested over this time for its durability and has been graded as a 'good resin' (Feller 1978; Feller 1994).
B72 has been used in glass conservation to consolidate paint layers, fix breaks, and protect crazed glass, for example on the windows in the Church of St Martha in Nuremberg (see section 5, Taylor 1984, and Marschner 1985a). Due to its durability, no changes in its molecular structure caused by light or weathering have been documented. The spectroscopic data derived from the film after natural weathering remain unchanged, as do its thermal qualities, physical qualities and solubility, to the extent that Paraloid B72 films remained soluble even after ageing with solvents of medium polarity. However, since the adhesion of this resin to the glass is relatively poor and dependent on the condition of the base glass, there were many cases where the film showed signs of loosening and flaking; this was particularly the case where the resin had been applied as a layer across a large area and was subject to tensile pressure. The film was particularly sensitive to attack from water, either rain or condensation, and tended to blister and flake off. For these reasons, it is recommended that Paraloid B72 is only used for the stabilization of paint contours after external protective glazing has been installed.
Another important acrylic resin in glass conservation is Plexidon M449, a polymethyl methacrylate. This product was combined with Vestinol C, a softening agent based on di-n-butylpthalate, for use in a plating technique developed by Richard Jacobi for the Cologne Cathedral workshop in the 1950s. The technique was used well into the 1980s, and the principles and implementation of this procedure, as well as the problems associated with it, have already been thoroughly discussed elsewhere (Brinkmann and Decker 1995). Here it will suffice to discuss the materials used for this plating procedure and their characteristics.
Plexidon M449 is a very chemically stable material, which dries to a solid, very flexible film. It softens at 109°C, its melting point. However, the mixture of this and the softening agent Vestinol C is soft, rubbery and sticky; its melting point, which depends on the concentration of softening agent, can be well below room temperature. This allows this system to be used as an adhesive in plating where solvent additives cannot be employed because the specific procedures would not allow the solvent to evaporate. A spectroscopic comparison of naturally weathered and new adhesive films confirmed the stability of this resin: the polymer had suffered no changes in its structure as a result of the ageing process, and even the addition of the softening agent did not affect its stability. Any optical changes, such as yellowing, browning and bubbling, can probably be traced either to reaction with the oils leached from the putty by the softening agent and absorbed in the film of plating adhesive, or to reaction with the adhesive originally used for break-fixing.
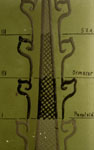
A dual-component adhesive based on polyethylenimin was developed by Jacobi at the Cologne workshop for use in break-fixing as part of his plating procedure (Schulz and Mehnert 1951). 6 This artificial resin was used as an adhesive for optical tools from 1950 to 1955, but after its withdrawal from the market (mainly on toxicological grounds) was specially produced for use by the cathedral workshop until 1978. Since for the present research only a very small number of samples of this adhesive (which had darkened, become brittle and decomposed extensively) were available, and since there was no reference material, no definite conclusions could be drawn as to this polymer's composition, and it was only possible to make theoretical suppositions on the polyethylenimin's ageing process and mechanisms. However, the latter are very probably the result of photochemical oxidization, leading to molecular disintegration and resulting in darkened and polarized fragments (fig. 2). This supposition would also explain why it was so unexpectedly easy to dissolve this product in water, which can be used to remove this adhesives or residues of it.
In principle therefore the plating procedures undertaken by Jacobi can still be reversed today, as despite the lapse of a number of years, the acrylic-resin film can still be dissolved quite easily in a solvent bath, without any mechanical stress to the glass surface or the paint applied to it. However, despite all disadvantages, particularly the loss of the original leading and the changes in the visual impact of the glass, there is no urgent reason to remove any plating until reliable alternatives have been developed. Removal of plating without first providing protection for a window against the weather, perhaps in the form of external protective glazing, is therefore not recommended.
Epoxy Resins
Together with acrylic resins, epoxy resins are the commonest artificial resins used in stained glass restoration. These are resins with multiple additives based on an epoxy and an amine. By varying the composition of the components, it has been possible to create a wide range of products, which have been used as glues, liners or laminates for industrial and technical purposes. Epoxies have also been widely used in conservation on account of their superior adhesive qualities and strength, for example as metal-glue, or for the conservation of archaeological objects, ceramics and stone (Selwitz 1992).
Epoxy resins have also been used since the beginning of the 1960s in the conservation of stained glass, mostly for break-fixing, but also for the targeted fixing of paint outlines, application over large areas, and plating (Weintraub and Greenland 1984; Bradley and Wilthew 1984). The big advantage of epoxy resins compared with the thermoplastic polymerization resins (polyvinyl acrylates and acrylic resins) is their greater adhesive strength and their ability to adhere well to the base layer. It has already been mentioned that such adhesive strength can be problematic, in cases where the strength of the external film exceeds that of the glass matrix as conserved. This can lead to secondary breaks adjacent to the glued area, or the flaking or peeling away of the gel glass layers with the layer of bonding agent on account of its tensile strength, all typical symptoms of the use of epoxy resins.
However, the biggest disadvantage of epoxy resins compared to polymer resins is their relatively poor durability. The reason for this is their polarized molecular structures: these can constitute a starting point for oxidation reactions, which are mostly photochemical in origin. This leads to yellowing or browning of the glass, often accompanied by changes in the physical characteristics of the film, which becomes considerably more fragile (Tennent 1979; Down 1986). This pronounced tendency of common epoxy resins towards yellowing is particularly noticeable where they have been used over a large area or as a plating adhesive. Most of the samples examined in this study were of these types, and were generally remarkable for the clear signs of ageing they exhibited (an overview of the types identified is given in table 1).
Most of these samples were identified as epoxy resins based on diglycidyl ether of bisphenol A (DGEBA) basis, an aromatic epoxy pre-polymer interlinked with amines. These were generally types of Araldite; both Araldite AY101 and AY103 are based on DGEBA, and are distinguished from each other by their molecular size and therefore viscosity. 7 It is recommended that both types be used with amine hardeners in order to control the speed at which the reaction resins set. Both types contain softening agents based on a di-n-butylphalate. Araldite XW396 is a newer product, in use since the 1970s. This contains a mixture of DGEBA and a mono-functional butyl glycidyl ether, a micro-molecular epoxy intended to act as a thinning agent. This softening-agent-free product is said to be less prone to yellowing, although this could not be confirmed in this research for naturally aged films of bonding agent.
Studies of light ageing have shown that epoxies with an aromatic pre-polymer like DGEBA tend to be vulnerable to oxidization, a tendency exacerbated by the presence of softening agents (Tennent 1979; Down 1986). Infra-red spectroscopic analysis of samples, both aged and otherwise, supported the descriptions of the reaction mechanisms found in the literature (Jägers and Brinkmann 1999).
Yellowing of Araldite-type epoxy resins was immediately observable in two cases, the Church of St John in Herford and the Minster in Mönchengladbach, where paint on windows was consolidated and large areas of glass covered with resin between 1970 and 1980. However, the condition of the glass and the paint layers was not a cause for concern. Comparisons with photographs taken before this treatment show that the lining is intact, apart from in a few small places, and the corrosion of the glass has been halted: it does not appear necessary therefore to undertake action to reverse the treatment. However, the use of such epoxy resins for plating in the early 1970s has proved much more problematic, for example in the case of the west window of Altenberg Cathedral (fig. 3). The artificial-resin film was peeling away and symptoms of moisture penetration could be observed. As a result of this, there was an obvious risk of micro-organisms multiplying between the glass and the resin film layer. It is therefore suggested that these layers be removed in the medium term, even though protective glazing has been installed for this Altenberg window.
Reversing a plating carried out with an acrylic resin is far more difficult than reversing treatments carried out with the acrylate resins described above. Epoxies are reactive resins and therefore effectively cannot be dissolved with organic solvents. Because at best they can only be swelled, and because they cannot be softened with heat, conservation treatment with them is considered irreversible. However, it is possible to remove the adhesive film with highly swelling solvents if the base layer is not porous or sensitive to organic solvents (von Derschau and Unger 1998). During the research undertaken for this study a process for dissolving epoxy-resin plating adhesive was developed, using a strongly polarized solvent called dimethyl sulfoxide applied in conjunction with a certain amount of heat (Jägers and Brinkmann 1999).
Together with the epoxy resins discussed above, other types of epoxy-resin products were also used, based on aliphatic pre-polymers; one example is the Hahn Cement 8 used to repair breaks, and another is Disbon, 9 which was used to cover large areas of nineteenth-century stained glass in the windows of Cologne Cathedral. In this case, dramatic damage to the surfaces of the stained glass was recorded. The thickly applied film of bonding agent had over time become extremely strong; as a result, tensions built up (particularly through the action of heat) that led to the film's peeling away, taking the layers of glass and paint that adhered to it with it. This example clearly demonstrates the great adhesive qualities and tensile strength of epoxy resins. In this particular example, the thickness of the layer applied exacerbated the effects of the resultant damage, but these products demonstrated the same qualities even in cases where the bonding agent had been applied relatively thinly. Disbon would appear from our spectroscopic analysis to be identical with a new product, HXTAL-NYL-1, which is currently recommended for use in glass conservation. 10
By contrast with the aromatic epoxies, aliphatic epoxies demonstrate greater strength and a lesser tendency to yellowing. Both of these qualities could be clearly demonstrated from the stained glass samples analyzed.
Polyesters
During the course of this study it was established that polyester resins have been used on occasion for the conservation of stained glass. These are dual-component glues based on an unsaturated polyester as pre-polymer, combined with Styrol. Spectroscopic analysis showed that these products were identical to Akemi, a product generally used by masons fr stone fixing. 11 During previous conservation, the details of which cannot be pinned down more closely, medieval stained glass from the collection in Löwenburg Castle near Kassel was treated with this material, which was used to repair breaks and spread a film over large areas. Once restored, the items were then stored for years in deposit in the castle. The ageing of the layer of adhesive cannot therefore be associated with the effects of the external climate. Yet there was evidence of change, with the adhesive becoming yellowed, brittle and flaky, and paint layers being lost or becoming unstable.
Since they are reactive resins, polyesters are in principle insoluble by organic solvents, and treatment with them is therefore considered, like that with epoxy resins, to be irreversible. However, in cases where the underlying layers of glass are not porous or sensitive to organic solvents, the same technique as was described above for removing plating adhesive from the Altenberg windows (the use of a particularly swelling solvent in conjunction with a certain degree of heat), could in principle be used to remove polyester resins.
Silicon Resins
Silicon resins, three-dimensionally combined polysiloxanes based on dialkyl- and trialkylsilanes, were generally used in architectural conservation or as a hydrophobizing agent in stone conservation. Since the corrosion processes in glass and stone are similar in many respects, and since both generally need to be protected from direct weathering, particularly from the effects of water, it appeared logical to experiment on stained glass with stone-conservation products. Experiments using silicon resins to treat stained glass and consolidate paint layers were conducted by the Cologne Cathedral workshop from 1975 to 1985. For this the dual-component product BS31 was mixed (2%) with the hardener TU2 12 and diluted with toluene. However, the effects of this agent have been proved to be negative, mostly because of its poor adhesion to the base layer of the glass. The extremely inflexible, tensile film layers break easily and lift from the surface in large flakes. The degree to which these negative effects were observed depended on the thickness of the film applied, with large-area application being more damaging than the application of small amounts for the consolidation of paint contours.
Analysis of these lifting films of bonding agent showed that there were no chemical changes in their structure, which again leads us to the conclusion that physical changes and poor adhesion to the glass base layer are the reasons for the damage. Although silicon resins are considered insoluble by organic solvents, again, it was found that the use of a range of solvents of varying polarity could be effective. Toluene, acetone and ethyl acetate were used. The surprising ease with which the films that had come away from the glass dissolved is difficult to explain: perhaps a particular mixture of resins and hardeners was used in which the components were not able to react as expected. Experiments at the Cologne Cathedral workshop with this product ceased in 1985. 13
Adhesive Strips and Foils
It is not uncommon to find self-adhesive strips on stained glass, generally applied to stabilize breaks and as a temporary safety measure during removal and transport. Virtually all studios make use of such materials for these purposes. Even temporary use can have negative consequences, particularly for aged glass and paint layers, especially if the adhesive bands are left on for weeks or months. An extreme example of application of adhesive foil over a large area of stained glass can be found on an armorial panel in the Historisches Museum der Stadt Köln (Historical Museum of the City of Cologne). The aim had been to stabilize the breaks and the dangerously fragile paint layers, and was clearly not intended as an emergency repair, but as a long-term measure.
There are two main categories of self-adhesive foils. In one of these groups the adhesive consists of a mixture of natural or synthetic rubber and a resin (turpentine resin, phenol resin, etc.); the other group makes use of permanently elastic acrylic-resin adhesives. The backing was composed of cellulose-acetate or cellophane foil. Softening agents based on phthalates were often added to the adhesive in both groups (O'Loughlin and Stiber 1992).
These two groups age differently. Over time, the rubber/resin masses oxidize easily, becoming yellowed and brittle, parting from the foil and sticking firmly to the glass. The acrylic-resin adhesives on the other hand do not oxidize so easily, though there is a danger that fluid elements may penetrate the porous layers beneath (the corrosion and gel layers). These areas become hydrophobic and appear discoloured or greasy; this is particularly noticeable under ultra-violet light. In the long term, the way in which these areas age or corrode will be noticeably different.
The same applies to larger-format self-adhesive foils, to which a very soft, rubbery elastic adhesive based on a polyvinyl-acetate-acrylate-co-polymer is applied, as in the case cited above. The same is true of self-adhesive labels, which are often applied to label panels, designate test areas, identify positions from which samples have been removed, etc. The adhesive strips and glue residues can be removed mechanically after treatment with organic solvents. The difficulty here lies in avoiding further damage to the already fragile paint layers or glass surface, though it is almost impossible to remove all residues from the adhesive and softening agent from very porous layers.
2.7. Assessment of the Research Results
During the course of this study of restored stained glass, evidence was found for the presence of an unexpectedly high number of products and techniques that are non-standard conservation, such as UHU glue or adhesive foils, which speaks clearly of unprofessional treatment of historical stained glass. However, the vast majority of the bonding agents used were tried and tested thermoplastics (polyvinyl acetates, acrylates such as polymethyl-methacrylate or methacrylic-acid-ester co-polymers), or duroplastics (epoxy resins).
It is notable that many of these materials have been used for the conservation of other materials, in particular ceramics and stone. Examples are the silicon resins used in buildings conservation or stone glues based on polyesters.
The condition of these naturally aged conservation agents from early restorations varied greatly, though they can be divided roughly into two groups. In the first group are those where the conservation agents have been radically affected over time by light, oxygen and humidity, becoming browned and brittle, or even disintegrating. In these cases, the agents have endangered the existence of the glass, as well as having an adverse visual (aesthetic) effect. This is mostly the case with the reactive resins (e.g., epoxy resins).
In many cases however the reason for the failure of these conservation materials was not their properties or durability, but rather a failure to adhere to the layer beneath or the surface of the glass. In the most fortunate cases, the materials had peeled away over time without damaging the surface, but in the worst cases, the stained glass paint and even entire layers of glass had been pulled away with them. Organic bonding agents are by their nature incapable of achieving a sufficiently strong adhesion to the glass, particularly when, like the polymerizing resins, they possess an non-polar molecular structure. In order to improve this situation, several authors have suggested adding adhesion facilitators and combining agents to glue or consolidators (Müller, Torge, Kruschke and Adam 1977; Errett, Lynn and Brill 1984; Weintraub and Greenland 1984). However, no such agents were identified during the course of the study, either through analysis or research. Furthermore, the research showed that damage occurred when these agents were applied in an uninformed manner, generally too thickly, not because of intrinsic characteristics of the agents. The reason for this is probably that conservators did not have enough information or experience relating to the use of these new, often complex, artificial-resin-based substances on historical stained glass.
In general, this study showed that the organic bonding agents previously used widely are only suitable for the conservation of stained glass within strictly defined conditions and circumstances, especially as far as weathered external surfaces are concerned. This is mainly because of the surfaces' vulnerability to the reactions of the ageing process caused by light and air, but also on account of the difference in the bonding agents' physical properties compared with glass. The main problems are their thermal and hygric properties, which can lead to tension within the layer of bonding agent. For this reason one should also consider introducing a protective external system, even when relatively durable substances such as the acrylic resin Paraloid B72 have been used.
The study also showed that the use of artificial resins in the conservation of stained glass is problematic and can be detrimental to the glass; nevertheless, they continue to be used today in the absence of alternatives. In order to achieve success in conservation, one should exercise care in selecting the correct approach to the problem, choosing conservation agents that are durable and appropriate for the particular situation; the results of experiments carried out in the laboratory during the course of this study will be helpful in this respect (see section 3 below). Of perhaps even greater importance than this is that the chosen substance be applied correctly, to a specification tailored to each individual case. The undesirable situations experienced when using of purely synthetic bonding agents in the conservation of stained glass have led recently to research into new possibilities. Two new substances have been developed: ORMOCER, 14 an organic modified silicate for application to glass surfaces (Fuchs, Römich, Leissner and Tur 1991), and Silicium-Zircon-Alcoxide SZA, for the stabilization of paint layers. Both have recently been successfully tested in pilot studies (see section 4).
3. Research into Known and Alternative Restoration and Conservation Agents
3.1. Introduction and Objectives
The research described below was conducted by Dr Hannelore Marschner and her colleagues at the Bayerisches Landesamt für Denkmalpflege (the Bavarian State Monuments Commission). The results are summarized here; the report has full details (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996). Damage to historic stained glass can vary widely and requires an appropriately specific response to its repair. The previous section explored the standards and principles to which materials used in glass conservation should conform, and differentiated between three main functions these materials should perform: crack repairing, paint-layer stabilization, and conservation of the surface of the glass. Marschner's study analyses and compares the various materials suitable and (in some cases) used for the conservation of glass today. The tests for the suitability and durability of these conservation materials can be divided into two sections: a comparison of conservation materials commonly used internationally, and a comparison of alternative materials.
Synthetic organic polymers, generally based on epoxy or acrylic resins, are presently employed for edge-bonding and paint consolidation. The study used samples of these substances provided by the studios, referred to henceforth as the 'studio samples'. The main aim of the laboratory experiments described below and of the analysis of the suitability and durability of these substances was to avoid damage to historical stained in the future glass through inappropriate use. A further aim was to compile an overview of those materials employed at present, and to present a series of comparative datasets.
The programme of analysis of these studio samples only covered some modern artificial resins. New products should therefore be developed in collaboration with the producers and suppliers of adhesives, lacquers and architectural conservation agents, and tested in the laboratory for their suitability and durability. These new products will be referred to as 'alternative glass conservation materials' or 'alternatives' for short. They will be considered under three main headings: those used for edge-bonding; those used for stabilizing paint layers; and those used for conserving the surface of the glass.
The best-known class of materials used for mending breaks are the dual-component epoxies, known for their strong adhesive properties, though also for their tendency to yellow. For this reason, new substances were compared with the older ones. A different class of material, the single-component adhesives that harden under ordinary light conditions or ultra-violet radiation, has been used only on occasion in the conservation of stained glass. Generally these adhesives have been used when mounting glass, but could provide an alternative to epoxies, if the results of tests for suitability and durability are positive. The speed at which they harden, a matter of minutes, can be determined with ultra-violet radiation. Other classes of material to be analyzed were silicon rubbers and cyanacrylates. The individual classes of synthetic resins have already been discussed in section 2.
Despite positive experiences with Paraloid B72, widely used as a paint consolidator, other new products would be tested and compared to see if the recently developed organic and silicium-organic products might possess better characteristics offering superior protection and stabilization potential (taking their intrinsic stability as a prerequisite). The suitability of these substances as paint stabilizers was also tested on simulated paint layers (Fuchs, Römich, Leissner and Tur 1991; Römich, Pilz and Fuchs 1993; Marschner, Bertelmann, Tilenschi, Koci and Schanz 1995).
An external protective-glazing system offers protection against the dangers of corrosion and is nowadays an efficient measure that can be applied almost anywhere: it prevents rain from reaching the external surface of the glass and prevents the formation of condensation for the most part. By contrast, it is now relatively rare to install a protective polymer layer, as their long-term effects of this are yet known and understood, and the possibility of corrosion continuing or increasing under the protective layer should not be underestimated. It is therefore not enough to test artificial resins that seem appropriate for ease of application and material stability: it is also necessary to test that these artificial resins in relation to the surface of the glass to establish if they are sufficiently stable under corrosive conditions in the presence of noxious substances, and if they protect the surface of glass from further corrosion.
An essential part of the assessment of artificial resins is the choice of methods suitable for such tests and experimentation. As with the earlier studies by the Bayerisches Landesamy für Denkmalpflege (Marschner 1985a), the materials tested here were examined for their intrinsic stability as well as their ability to adhere to the surface of the glass by subjecting them to simulated natural conditions in a climate chamber, exposing them to varying degrees of temperature, humidity and ultra-violet light. In order to assess a material's conservation potential (its ability to protect the glass), one has to expose corrosion-sensitive model-glass sensors to the damage-causing agents found in the environment in order to simulate weathered conditions in a short space of time.
For the purpose of this research, model glass of medieval chemical composition was blown into sheets, and a 'standard corrosion procedure' was developed especially (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996, part 1). The stages of this procedure, which could be repeated cyclically, are as follows: covering the model glass with a thin film of artificial resin, moistening it with a mixture of sulphur and saltpetre, then subjecting it to climate change stress in a climate chamber. The acidic mixture was intended to simulate the gases SO2 and NOx (Löbel 1988). The major advantage of this standardized corrosion process is using the quantities of calium and calcium leached as a measure of the progress of corrosion (and therefore the protective potential of the conservation material). Whether this 'standard corrosion procedure', though being relatively simple in conception, is a suitable substitute for subjecting model glass to the action of noxious gases in the climate cabinet can only be answered by comparing the two techniques.
The results of these tests on conservation materials were divided into three groups according to their function (break-repairers, paint stabilizers, surface conservers), and are summarized here in accordance with various criteria and presented in numeric form using an assessment procedure specially developed for the purpose. The characteristics of the materials were analyzed before and after the corrosion procedure. These figures will be compared with one another using defined algorithms. The 'final marks' obtained in this way for various characteristics can be used as semi-quantitive values when comparing the suitability and durability of the materials tested.
3.2. Conventional Materials Commonly Used in Conservation Practice
The following glass conservation studios produced samples (see fig. 5) to a precise specification for the purposes of this research: Munich, Cologne, Berlin, Linnich, Canterbury and Lincoln. The materials chosen (see tables 2 and 3) were applied to small panes of clear modern sheet glass measuring 3cm by 5cm. Irrespective of their intended conservation function, all materials were applied in a thin layer to the samples in order to gain an understanding of their liability to become crazed or yellowed. In addition, the value of named adhesives for edge-bonding and plating was tested. The compatibility of various materials at the point where they come into contact, an important issue, was also tested by combining samples.
| Designation | Designation |
|---|---|
| Araldite AY103/HY956 | epoxy resin |
| Araldite AY103/KY056 with black pigment | epoxy resin + pigment |
| Araldite XW39/XW397 | epoxy resin |
| Araldite XW39/XW397 with black pigment | epoxy resin + pigment |
| epoxy resin BK/hardener BK | epoxy resin |
| HXTAL NYL-1 | epoxy resin |
| Hahn glass cement SH 1 black | epoxy resin + pigment |
| Hahn glass cement SH 1 black | epoxy resin + pigment + quarz |
| Agomet 456S/hardener | reactive acrylic resin |
| Conloc 651 | urethanmethacrylate + UV |
| Conloc 630 | modified acrylic resin + UV |
| Conloc 660 | modified acrylic resin + UV |
| Conloc 665 | modified acrylic resin + UV |
| Conloc 682 | modified acrylic resin + UV |
| Conloc 682 + activator 953 | modified acrylic resin + UV |
| ELC 4481 | modified acrylic resin + UV |
| Loctite glass adhesive | modified acrylic resin + UV |
| NOA 61 optics adhesive | modified acrylic resin + UV |
| Vitralit 6127 | modified acrylic resin + UV |
| Vitralit 6128 | modified acrylic resin + UV |
| Vitralit 7256 | modified acrylic resin + UV |
| Vitralit 7515 | modified acrylic resin + UV |
| Conloc SK 713 2K silicon | silicon latex |
| Durasil L Aquarium adhesive | silicon latex |
| Elastosil E 43 | silicon latex |
| Novasil S 28 | silicon latex |
| EGO-Silicon 200 | silicon latex |
| EGO-Silicon 110 | silicon latex |
| Silicon CAF 3 THIXO | silicon latex |
| Silicon CAF 3 THICO + CAF 33 NOIR | silicon latex |
| Silicon CAF 33 NOIR | silicon latex |
| Bindulin 2000 | cyanoacrylate |
| UHU K 3000 | cyanoacrylate |
| Designation | Type of material |
|---|---|
| beeswax | wax |
| beeswax + 5% NB 1115 | wax |
| beeswax + carnauba wax (60/40) | wax |
| carnauba wax | wax |
| Paraloid 5% in butyl acetate | non-reactive polyacrylate |
| Paraloid 5% in ethyl acetate | non-reactive polyacrylate |
| Paraloid 5% in toluene | non-reactive polyacrylate |
| Paraloid 5% in toluene/white spirit (8:2) | non-reactive polyacrylate |
| Paraloid 7.5% in ethyl acetate | non-reactive polyacrylate |
| Paraloid 7.5% in toluene | non-reactive polyacrylate |
| Paraloid 7.5% in toluene/white spirit (8:2) | non-reactive polyacrylate |
| Paraloid 10% in toluene | non-reactive polyacrylate |
| Paraloid 20% in toluene | non-reactive polyacrylate |
| Paraloid 30% in toluene | non-reactive polyacrylate |
| Silicon modeller NM 327 with hardener/benzine (1:1) | organically modified silicon |
| ORMOCER/Paraloid (50/50) / 1:10 ethyl acetate | heteropolysiloxane / acrylic resin |
| SZA (silicium zirconium alkoxide) | silica ester |
| Kallocyl CP-GM | polymer + pigment |
Adhesives Commonly Used for Edge-Bonding
Among the conservation materials commonly used by studios for fixing breaks, epoxy resins, ultra-violet-reactive acrylic resins, silicon rubber, and so-called instant glues were chosen for analysis. Epoxy resins are most often used, because of their strength and long-lasting adhesion. Under ultra-violet radiation all varieties of this type of material had a tendency to yellow. This yellowing is however a minor disadvantage in narrow cracks. The epoxy-resin glue HXTAL NYL-1, mainly used in the USA and England, showed the least tendency to darken, but proved to be particularly strong and thus inflexible (see section 2). The commonly used adhesive Araldite AY103/HY956 should be replaced by Araldite XW396/XW397. Ultra-violet-reactive resins are also liable to darken to varying degrees, though during tests the optics adhesive NOA61 proved to be the least affected (Sander-Conwell and Schmidt-Ott 1993). Silicon-rubber materials proved to be very stable under simulated environmental conditions. However, their optical and physical qualities are unsuitable for break-fixing, because they are extremely viscous, cloudy and also too soft. The fact that these materials are insoluble (i.e., cannot be removed with solvents), is a further disadvantage in the use of this class of material for the conservation of historical stained glass. Though instant glues, usually cyanacrylates, do not tend to yellow, their adhesion to the glass is very poor.
Self-adhesive foils are often used on stained glass to stabilize breaks as a temporary safety measure during removal and transport. The simulated environmental tests showed that they could be very unreliable, depending on the particular type. Destructive deposits of dirt can build up if great care is not taken in removing the foils or any residues of the adhesive.
Common Paint-Layer Consolidators
Both in the past and now, waxes, acrylic resins and, less commonly, silicium-organic products have been used to stabilize paint layers. The characteristics of the waxes depend greatly on the temperature: at low temperatures they become brittle and prone to cracking, at higher temperatures they become softer and they close up any gaps that may have formed. They are also opaque and not colourless. During the simulated environmental tests, they showed no signs of yellowing. Among the polyacrylates, Paraloid B72 is commonly used in several other conservation disciplines. It has been used in stained glass conservation for over ten years to consolidate loose paint layers (Marschner 1985a; Müller-Weinitschke 1995). The tests confirmed Paraloid B72's excellent ability to resist weathering, though its ability to adhere to the surface of the glass decreased noticeably under wet weathering conditions.
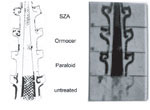
Silicium-organic substances, like silicium-zirconium-alkoxide (SZA), which has been introduced into modern conservation practice on an experimental basis, are intended for use on powdery paint layers, and should only be applied in highly dilute form. When applied thickly, they sometimes proved to adhere insufficiently to the glass (Fuchs, Römich, Leissner and Tur 1991; Römich, Pilz and Fuchs 1993).
The various stages of the research and test process are shown in fig. 6. To ensure that the materials on the samples had hardened completely, the tests for stability were carried out in the climate chamber no less than four weeks after the resins had been applied. The samples were photographed before, during and after the climate simulation programmes I and II, and were analyzed for transparency, colouring, surface characteristics, and the mechanical stability of the break fix. These observations were taken in shorthand and summarized in accordance with a simplified assessment procedure.
Mutual Tolerance of Various Materials
The combined samples tested exhibited no mutual intolerance during the weathering period. Experience in practice, however, has shown that instances of intolerance indeed occur, for example the mutual dispersion of parts of the artificial resins with low molecular weight (catalysts, softening agents, etc.). These changes in the characteristics of the material can result in a heightened tendency to yellow and crack.
3.3. Alternative Materials for the Conservation of Stained Glass
Edge-bonding Adhesives
In addition to the groups of materials commonly used in workshop practice, research and testing was carried out in the laboratory on other types of break-fixer. In order to test their physical stability, the materials were spread on a colourless sheet of glass (48cm by 27 cm and 1mm thick) with a brush or spatula. Two such sheets were glued together edge to edge to simulate a break-fix. The diagram in fig. 7 illustrates the process. The transparency, colouring, surface characteristics, and the mechanical stability of the break-fix were analyzed before, during and after climate simulation programmes I and II, as well as after the ultrasound test. The observations were summarized in a grading diagram.
The general material characteristics of these product groups were corroborated in these tests. Among other things, the merits of epoxy-resin glue Araldite XW396/XW397 compared with the other Araldite products tested were confirmed. A special series of test was undertaken to increase the viscosity of this very fluid adhesive by adding highly dispersive silicic gels (Aerosil 90 and 300, 1-5% by weight). Tests on the material's stability showed slightly decreased levels of adhesive strength in combination with increased levels of additives. Of the ultra-violet-reactive adhesives tested, Conloc UV651 achieved the best overall results.
Daylight-reactive adhesives, which harden in the visible light spectrum, have been developed recently. However, in their current state of development their adhesive and cohesive qualities are not yet satisfactory for fixing breaks in glass. Silicon rubbers are generally highly stable, but are not suitable for break-fixing for the reasons mentioned above, remaining milky in appearance even when carefully prepared. An exception is Delo Gum VT 3198, which remains clear. The adhesives tested are collated in table 4.
| Type of adhesive and product name | Producer | Time-span available to manipulate / time taken to harden | Appearance as supplied by the producer (refraction index) | Viscosity (in millipascals) | Remarks | Condition and appearance before treatment / after treatment in the climate chamber | Adhesion before treatment / after treatment in the climate chamber / after the acid test | Condition and appearance before treatment / Condition and appearance after treatment in the climate chamber | Adhesion before treatment / Adhesion after treatment in the climate chamber / ultrasonic test |
|---|---|---|---|---|---|---|---|---|---|
| Araldite AY103/HY956 | Ciba Geigy | 2.5 hours / 2 days | yellowed | slightly viscous to viscous (850-1400) | 5 / 3 (yellowed) | 4 (cracks in the coating) / 5 / 5 | 5 / 4 | 5 / 5 / 5 | |
| Araldite AY103/HY956 | Ciba Geigy | 2.5 hours / 2 days | yellowed | slightly viscous to viscous (850-1400) | (results when applied after a longer period of mizing) | 5 / 4 (yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 |
| Araldite AY103/HY951 | Ciba Geigy | 2.75 hours / 7 days | yellowed | slightly viscous (700-1100) | 5 / 4 (yellowed) | 4 (cracks in the coating) / 5 / 2 | 5 / 5 | 5 / 5 / 5 | |
| Araldite BY158/HY2992+2996 | Ciba Geigy | 25 mins / 2 days | no information | slightly viscous | 5 / 5 | 5 / 5 / 4 | 5 / 5 | 5 / 2 / 1 | |
| Araldite BY158/HY2996 | Ciba Geigy | 50 mins / 2 days | no information | slightly viscous (150) | a thin liquid | 5 / 5 | 5 / 5 / 4 | 5 / 5 | 5 / 2 / 1 |
| Araldite XW396/XW397 | Ciba Geigy | 40-50 mins / 12-15 hours | clear (n = 1.55) | slightly viscous (150) | a thin liquid | not tested | not tested | not tested | not tested |
| Araldite XW396/XW397 | Ciba Geigy | 40-50 mins / 12-15 hours | clear (n = 1.55) | slightly viscous (150) | a thin liquid | 5 / 4 (yellowed) | 5 / 5 / 5 | 5 / 4 (yellowed) | 5 / 5 / 5 |
| Araldite XW396/XW397 | Ciba Geigy | 40-50 mins / 12-15 hours | clear (n = 1.55) | slightly viscous (150) | a thin liquid (results after additional hardening at 50°C) | 5 / 4 (yellowed) | 5 / 5 / 5 | 5 / 4 (yellowed) | 5 / 1 / not tested |
| Araldite XW396/XW397 | Ciba Geigy | 40-50 mins / 12-15 hours | clear (n = 1.55) | slightly viscous (150) | a thin liquid | 5 / 4 (yellowed) | 5 / 3 (cracks in the coating) / 4 | 5 / 4 (yellowed) | 5 / 3 / 5 |
| Araldite XW396/XW397 | Ciba Geigy | 40-50 mins / 12-15 hours | clear (n = 1.55) | slightly viscous /viscous | a thin liquid (results with thin coating) | 5 / 4 (yellowed) | 5 / 5 / 4 | 5 / 4 (yellowed) | 5 / 3 / 5 |
| Araldite XW396/XW397 (1% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | slightly viscous /viscous | 1% additive SiO2 (90m2 per gram) | 5 / 4 (yellowed) | 5 / 3 (cracks in the coating) / 2 | 5 / 5 | 5 / 5 / 1 |
| Araldite XW396/XW397 (1% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | viscous | 1% additive SiO2 (90m2per gram) (results with thin coating) | 5 / 5 | 5 / 5 / 4 | 5 / 5 | 5 / 5 / 1 |
| Araldite XW396/XW397 (3% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | viscous | 3% additive SiO2 (90m2 per gram) | 4 (cloudy on account of air inclusions) / 4 (yellowed) | 5 / 4 (break not mended as crack not sufficiently filled / 5 | ||
| Araldite XW396/XW397 (3% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | very viscous | 3% additive SiO2 (90m2 per gram) (results with thin coating) | 4 (cloudy on account of air inclusions) / 5 | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 |
| Araldite XW396/XW397 (5% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | very viscous | 5% additive SiO2 (90m2 per gram) | 4 (cloudy on account of air inclusions) / 4 (yellowed) | 5 / 4 (break not mended as crack not sufficiently filled) / 5 | 5 / 5 | 5 / 5 / 5 |
| Araldite XW396/XW397 (5% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | slightly viscous /viscous | 5% additive SiO2 (90m2 per gram) (results with thin coating) | 5 / 3 (yellowed and cloudy on account of air inclusions) | 5 / 5 / 5 | 5 / 5 / 5 | |
| Araldite XW396/XW397 (1% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | slightly viscous /viscous | 1% additive SiO2 (300m2 per gram) | 5 / 5 | 5 / 4 (stress crack in the glass) / 4 | ||
| Araldite XW396/XW397 (1% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | viscous | 1% additive SiO2 (300m2 per gram) (results with thin coating) | 4 (cloudy on account of air inclusions) / 4 (yellowed) | 5 / 5 / 4 | ||
| Araldite XW396/XW397 (3% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | viscous | 3% additive SiO2 (300m2 per gram) | 5 / (not tested) | 5 / (not tested) / 5 | 5 / (cloudy on account of air inclusions) / 5 | 4 / (blistering, poor wetting, considerable shrinkage and consequent poor filling of crack) / 5 / 3 |
| Araldite XW396/XW397 (3% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | very viscous | 5% additive SiO2 (300m2 per gram) (results with thin coating) | 5 / 4 (yellowed) | 5 / 4 (stress crack in the glass) / 5 | 5 / (cloudy on account of air inclusions) / 5 | 5 / 5 / 1 |
| Araldite XW396/XW397 (5% Aerosil 90) | Ciba Geigy (Degussa) | no information / ~20 hours | no information | very viscous | 5% additive SiO2 (300m2 per gram) (results with thin coating) | 5 / (not tested) | 5 / (not tested) / 5 | 5 / (cloudy on account of air inclusions | 5 / 5 / 1 |
| Delopox VP02 | Delo | 90 mins / 20 hours | pale yellow-brown | viscous (2500) | 4 (yellowed) / 5 | 5 / 2 (cracks in the coating) / 5 | 5 / 5 | 5 / 2 / 3 | |
| Delopox VP02 | Delo | 90 mins / 20 hours | pale yellow-brown | viscous (2500) | (results with a longer mixing period) | 4 / 5 | 5 / 5 / 5 | 5 /5 | 5 / 2 / 3 |
| Delopox 1892 | Delo | 1 hour / 24 hours | yellowed | extremely viscous (50000) | extremely viscous | 4 (cloudy, yellowed) / 5 | 5 / 5 / 4 | 4 (cloudy, yellowed) / 5 | 5 / 5 / 5 |
| Type of adhesive and product name | Producer | Time-span available to manipulate/ time taken to harden | Appearance as given by the producer | Viscosity (in millipascals) | Remarks | Condition and appearance before treatment/after treatment in the climate chamber | Adhesion before treatment / after treatment in the climate chamber / after the acid test | Condition and appearance before treatment / Condition and appearance after treatment in the climate chamber | Adhesion before treatment / Adhesion after treatment in the climate chamber / ultrasonic test |
|---|---|---|---|---|---|---|---|---|---|
| Delomet 2301 (acetate) | Delo | 20 mins / 2mm in 24 hours | milky, transparent | pliable (350000) | elastic | 5 / 5 (slightly yellowed) | 5/5/5 | 5/5 | 5 / 5 / 5 |
| Delo Gum 3479 (oxim) | Delo | 10 mins / 4mm in 24 hours | milky, transparent | very viscous (10000) | very elastic | 4 (cloudy) / 5 (slightly yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 |
| Delo Gun VT 3198 (acetate) | Delo | 10 mins / 2mm in 24 hours | clear | (no details) | not milky | 4 (cloudy) / 5 (slightly yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 |
| Type of adhesive and product name | Producer | Time-span available to manipulate / time taken to harden | Appearance (as given by the producer) | Viscosity (in millipascals) | Remarks | Condition and appearance before treatment / after treatment in the climate chamber | Adhesion before treatment / after treatment in the climate chamber / after the acid test | Condition and appearance before treatment / Condition and appearance after treatment in the climate chamber | Adhesion before treatment / Adhesion after treatment in the climate chamber / ultrasonic test |
|---|---|---|---|---|---|---|---|---|---|
| UHU Sekundenkleber (instant glue) | UHU | - / approx. 5 seconds | colourless | very slightly viscous | 5 / 5 | 5 / 1 / (not tested) | 5 / (not tested) | 5 / 1 / (not tested) |
| Type of adhesive and product name | Producer | Time-span available to manipulate / time taken to harden | Appearance (as given by the producer) | Viscosity (in millipascals) | Remarks | Condition and appearance before treatment / after treatment in the climate chamber | Adhesion before treatment / after treatment in the climate chamber / after the acid test | Condition and appearance before treatment / Condition and appearance after treatment in the climate chamber | Adhesion before treatment / Adhesion after treatment in the climate chamber / ultrasonic test |
|---|---|---|---|---|---|---|---|---|---|
| Photobond 400 | Delo | (no details)/(no details) | colourless, clear | very viscous (3500) | brittle | 5 / 5 (yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 1 (brittle) / (not tested) |
| Photobond 4442/1 (covered with a foil covering when exposed to light) | Delo | (no details)/(no details) | yellowy | slightly viscous (650) | very elastic | 5 (cloudy) / 5 | 5 / 5 / 1 | 5 / 5 | 4 (blistering, poor wetting, considerable shrinkage and consequent poor filling of crack) / 3 (blistering, poor wetting, considerable shrinkage and consequent poor filling of crack) / (not tested) |
| Photobond 4442/1 (covered with a foil covering when exposed to light) | Delo | (no details)/(no details) | yellowy | slightly viscous (650) | very elastic (results after intensive exposure) | 5 (cloudy) / 5 | 5 / 1 / 1 | 5 / 5 | 4 (blistering, poor wetting, considerable shrinkage and consequent poor filling of crack) / 3 (blistering, poor wetting, considerable shrinkage and consequent poor filling of crack) / (not tested) |
| Type of adhesive and product name | Producer | Time-span available to manipulate / time taken to harden | Appearance (as given by the producer) | Viscosity (in millipascals) | Remarks | Condition and appearance before treatment / after treatment in the climate chamber | Adhesion before treatment / after treatment in the climate chamber / after the acid test | Condition and appearance before treatment / Condition and appearance after treatment in the climate chamber | Adhesion before treatment / Adhesion after treatment in the climate chamber / ultrasonic test |
|---|---|---|---|---|---|---|---|---|---|
| Conloc UV651 | EGO | (no details) / (no details) | transparent (n=1.48) | very viscous (4500) | 5 / 5 (slightly yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 | |
| Conloc UV665 | EGO | (no details) / (no details) | transparent (n=1.55) | slightly viscous (200) | 5 / 3 (slightly yellowed) | 5 / 5 / 5 | 5 / 4 (yellowed) | 5 / 5 / 5 | |
| Conloc UV682 | EGO | (no details) / (no details) | transparent (n=1.5) | slightly viscous (800) | 4 (cloudy) / 4 (yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 | |
| Loctite 350 | Loctite | (no details) / (no details) | clear | very viscous (3000-6000) | 4 (cloudy) / 5 (yellowed) | 5 / 5 / 5 | 5 / 5 | 5 / 5 / 5 | |
| Photobond 315 | Delo | (no details) / (no details) | colourless, clear | very slightly viscous (70) | particularly runny (results after cleaning with isopropanol) | 4 (surface sticky on account of the inhibiting effect of oxygen) / 5 | 5 / 5 / 1 | 5 / 5 | 5 / 5 / 5 |
| Photobond 315 | Delo | (no details) / (no details) | colourless, clear | very slightly viscous (70) | particularly runny (results after exposure to ultraviolet light in the presence of inert gas) | 4 (surface sticky on account of the inhibiting effect of oxygen) / 4 (yellowed) | 5 / 5 / 1 | 5 / 5 | 5 / 5 / 5 |
| Photobond 315 | Delo | (no details) / (no details) | colourless, clear | very slightly viscous (70) | particularly runny (results after exposure to ultraviolet light under protective foil) | 4 (cloudy) / 5 | 5 / 5 / 1 | 5 / 5 | 5 / 5 / 5 |
| Lotos 2 | Delo | (no details) / (no details) | transparent, clear (n = 1.52) | very slightly viscous (20) | particularly runny (results after cleaning with isopropanol) | 4 (surface sticky on account of the inhibiting effect of oxygen) / 5 | 5 / 5 / 3 | 5 / (not tested) | 2 / (not tested) / (not tested) |
| Lotos 2 | Delo | (no details) / (no details) | transparent, clear (n = 1.52) | very slightly viscous (20) | particularly runny (results after exposure to ultraviolet light under protective foil) | 5 (cloudy) / 5 | 5 / 5 / 3 | 5 / (not tested) | 2 / (not tested) / (not tested) |
| Optik-Kleber NOA61 | Norland | (no details) / (no details) | clear (n=1.56) | slightly viscous (300) | 5 / 5 | 5 / 5 / 3 | 5 / 5 | 4 / 5 / 5 | |
| Optik-Kleber NOA61 | Norland | (no details) / (no details) | clear (n=1.54) | very viscous (5000) | 5 / 5 | 5 / 5 / 3 | 5 / 5 | 4 / 1 / 1 |
Key
- very poor
- poor
- moderate
- good
- very good
Paint-layer Consolidators
The testing of alternative materials as paint-layer consolidators took up the greater part of this study. A large number of materials in various formula combinations were analyzed during the course of a comprehensive programme of research and testing, and selected after specific stages in the test series. In this way weaknesses could be recognized early on, the formulas changed, and specific variants of the materials with optimized characteristics found. The results of theses tests were assessed constantly in comparison with Paraloid B72, widely used in conservation as a paint-layer consolidator. Further series of tests were carried out to see if the qualities of Paraloid B72 could be improved with additives or adhesion facilitators, and whether there was a viable alternative to Paraloid B72.
Test samples and commercial products donated by several different manufacturers were selected for comparative research. For the majority of these artificial resins there was no experience of their use on glass, so it was necessary to modify some of the components and adjust the application process. The components of the products were only known in broad outline, as the manufacturers provided no details regarding additives (softening agents, photo-inhibitors, etc.). The following products were tested.
- stone-protection agents based on polysiloxane (SI)
- building protection agents produced by the firm Wacker (Wacker 270, 280, 550, etc.); mixtures of alkoxysilanes and alkyl alkoxysilanes, mainly dimethyl diethoxylane (DexDMS) and propyl triethoxylane (PrTExS) (Wendler, Klemm and Snethlage 1990).
- stone-protection agents based on polyurethane (PUR): Raboseal (a single-component polyurethane resin produced by the firm R+S), with a pre-polymer (isocyanate-modified polydimethyl siloxane, produced by the firm Bayer)
- lacquer based on polyurethane (PUR): various resin types from the Desmophene product series, for example Desmophene A160 (Bayer) and hardener Desmodur N75 (henceforth shortened to DD)
- lacquer based on alkyde resin (alkydes): alkydes that had been rendered hydrophobic with the silicon intermediary Silres SY (produced by the firm Wacker)
- artificial resins based on acrylic (acrylates): Paraloid B72 (produced by the firm Rohm & Haas), in both its original and modified forms
- glues based on epoxy resin (EP): various types of Araldite (produced by the firm Ciba-Geigy) and industrial glues produced by the firm Delo
- glues based on silicon resin (SI), e.g., the silicon resin Delomet 2301.
These materials were mainly single- or dual-component reactive formulations. The components harden on contact with humidity, oxygen in the air, or through the addition of amine hardeners. Only the fully pre-polymerized Paraloid B72 hardens through evaporation of the solvent.
The standard trade products were modified for use on glass, for example by being thinned with various organic solvents, or by adding catalysts or substances that reacted when on the surface of the glass. Various silane adhesion-promoting agents were added to improve adhesion to the glass, for example γ-amino propyl trimethoxysilane (Ha, produced by Union Carbide), or N-β-amino-ethyl-γ-amino-propyl trimethoxylane (Ha', produced by the firm Wacker). In order to achieve hydrophobization of the artificial resins, ailane and siloxane products (for example Wacker 280 and various types of Silres SY) were added. To achieve hydrophobization by a different methods, BS31 (produced by the firm Wacker) was applied as a coating layer. The large number of materials and combinations of materials tested cannot be described in detail here, but are fully documented with data sheets in the final report (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996, part 2).
Three types of sample were used for the laboratory experiments.
- untreated and keyed test-plates, to which the artificial resins were applied with pipettes or brushes, or by immersion
- corrosion-sensitive model glasses, produced with traditional techniques by the cylinder process at the Lambert antique glass studio in Waldsassen; the glass components and their corrosion profile are described in the final report (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996, part 1). The glass was pre-corroded and immersed in the test material.
- painted test-plates or model glasses, onto which outlines were painted in stained glass paint and dried on loosely (1 hour at 400°C). The artificial-resin solutions were stippled on with a brush.
The artificial resins in the various series of samples were tested according to various criteria. The over-arching criterion in testing was the materials' fundamental suitability, i.e., the ease with which the artificial-resin solution could be worked, the hardness and qualities of the film formed, its stability, protective effects and consolidating potential. During the tests individual samples were analyzed for such qualities as colour, transparency, glossiness, adhesion, and lack of defects and cracks, as well as their ability to retain these qualities. Other criteria for the effectiveness of the protection afforded by artificial-resin films on model glasses subjected to speeded-up corrosion processes were the decrease in damage to the surface of the glass, the decrease in the build-up of secondary corrosive products, and reduced leaching of components of the glass.
To achieve this a 'standard corrosion process' was developed (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996, part 1). The quantities of calcium and potassium leached from treated model glass were compared with an untreated reference sample, and constituted a quantitive measure of the resin's protective effect. This test procedure subjected the resin film to considerable corrosive attack. On the other hand, weathering with noxious gases in the climate chamber took twice as long to achieve the same level of corrosion and subjected the film to milder corrosion by comparison.
Comparison of the results of individual parts of the test series was made much easier by the use of a specially developed marking algorithm to create an overview of numerous individual observations. Fundamental suitability, stability and protective effect were given a mark out of 5 under 21 individual criteria. The ability of the artificial resins to consolidate porous paint layers was assessed under four further individual criteria. The results of the individual tests can be found in the final report (Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996, part 2).
Out of the range of material formulations tested at individual stages of the testing process, it became clear that of the tested materials only the products such as polyurethane lacquers and the acrylic resins were suitable for paint-layer consolidation. Formulations with added polysiloxanes, or protective polysiloxane films heightened the artificial resins' hydrophobic tendency. Treatment of the surface of the glass with the adhesion promoter γ-amine-primyl trimethoysilane (Ha) invariably meant that the ability of the coating layers to adhere, both initially and in the longer term, was improved. The hydrophobizing component Silres SY202 (a polysiloxane produced by the firm Wacker) proved to be compatible with the polyurethanes; however, in the tests for stability, it showed itself to be slightly incompatible when combined with the polyacrylate Paraloid B72. The advantage of increased hydrophobization -the reduction in both the amount of the surface exposed to condensation containing noxious substances and the length of that exposure - was not seen to improve the protective effect offered under these conditions to any noticeable degree.
Weighing up the results of these studies and also the advantages and disadvantages of the various products (such as ease of handling, reversibility, ease of storage etc.), the following formulations achieved the best results of the materials tested: Ha/Paraloid B72; Ha/Paraloid/B72/Silres SY201; Desmophen A160/Desmodur N75 (DD); and Ha/DD/Silres SY201. When one also takes into account visual impression, adhesive ability, stability and the protective potential of the film, the polyurethanes, particularly Ha/DD, are the best. However, the irreversibility of dual-component interlinking polyurethanes is a big disadvantage. Paraloid B72, even when modified with Silres SY201, is soluble in organic solutions, and hardened masses Paraloid can be regenerated through softening with solvents.
The application and removal of adhesive foils to test the degree with which paint contours adhered to the model-glass samples showed no significant variations between the various optimized formulations. Minor differences can be regarded as only tendential in test series such as these.
The consolidating effects of the various polysiloxanes were seen to vary more. Given their chemical affinity, one would expect excellent adhesion to the surface of the glass. However, when applied in thick layers, this class of product tended to produce a crazed film on account of its brittleness. This effect was seen both with the workshop sample (SZA) and with the alternative materials tested. Because of these physical characteristics, the concentration, thickness and method of application should be carefully tailored to the task in hand.
Before these materials are introduced into conservation practice, however, their functional suitability, ease of handling and durability must be tested in pilot studies under studio conditions and with exposure to natural weathering. The laboratory tests with their artificially speed-up weathering conditions only allow relative comparisons between the laboratory samples to be made, and conclusions should not be applied directly to real-life weathering processes (Helmen and Fakhoury 1993).
Conservation Materials
The conservation of eroded glass surfaces with coatings of artificial resin has been under discussion and examination ever since these materials were introduced, and experience with them has given cause for alarm on several occasions (see section 2). The main reasons for this are the poor reversibility of such measures, the insufficient levels of protection afforded, and the consequent damage to works of art. As a result of the research, three quite separate approaches to the protection of the surfaces of glass, which could be applied when testing the suitability of artificial resins, were developed.
- Conservation through coating. For the purposes of this report, this means coating with lacquer-like layers of organic or silicium-organic artificial resins that dry to a thickness of 1-50μm, stabilize an eroded gel-layer in the glass, and protect it from further corrosion.
- Conservation through thin-film coating. A very thin layer (less than 1μm thick) of a suitable hydrophobizing agent is applied to the surface of the glass. These thin films possess no stabilizing qualities, relying entirely for their protective effect on the reduction of the area exposed to condensation containing noxious substances and the length of that exposure.
- Conservation through diffusion-barriers. In this technique, the surface of the glass is coated with one or more layers of substance, at least one of which is has diffusion-barring characteristics. This aims ideally to prevent noxious gases from penetrating the surface of the glass completely.
The ability to these materials to protect against corrosion is assessed in what follows, as are the visual impression of the final result, the results of the adhesion tests, and the durability of samples subjected to speeded-up corrosion in the climate chamber. The conservation materials were tested with the same techniques and criteria as the paint-consolidation agents. The same materials tested for their ability to consolidate paint were also selected as lacquer-like products for layer-coating (see above). The following assessment from the results obtained can be summarized as follows: the materials that achieved the best results for initial and longer-term adhesive qualities, durability, appearance, and level of protection afforded wereParaloid B72; Ha/Paraloid B72; Ha/Paraloid B72/Silres SY201; DD; Ha/DD; and HA/DD/Silres SY201. Since these combinations proved themselves suitable for consolidating paint layers on model-glass samples, it could be expected that they will also lend themselves to stabilizing eroded, rough and unstable glass surfaces (gel-layers).
Layer-coating pre-corroded model glass produced the following results. Morphologically critical structures (such as cracks in the leached surface of the glass, protruding swellings, or secondary corrosion products) were generally very well stabilized by these products, with the exception of a few highly critical areas where faults in the coating could be observed arising from the accumulation of corrosion salts and the products of glass damage. The protective effect of these coatings, measured by the reduction in leaching (20% on average) should be scrutinized carefully, as a build-up of secondary corrosion products was observed between the surface of the glass and the coating. These corrosion products are potentially damaging for both the artificial-resin coating and the worn surface of the glass, since the growth of crystals damages the coating and creates mechanical tensions within cracks. The growth of the crystals proved that the corrosion processes were continuing, although at a much reduced speed, and there was no evidence that they increase the speed of corrosion. It is not possible to recommend lacquer-like materials for the conservation of glass surfaces in their present stage of development, as they do not afford sufficiently high levels of protection, and negative effects can be expected further down the line.
Thin-film conservation of the surfaces of glass by immersion in alkyl-silane solutions and subsequent rinsing increased the glass's hydrophobic tendencies. Visually, the films of these materials were completely neutral and fleck-free, both before and after testing in the climate chamber. Despite the thinness of the film, its protective effect was remarkable, reducing leaching by an average of 70%; corrosion damage to the surface of the glass was also reduced. Secondary corrosion products only crystallized in a few very obviously damaged areas. Among the materials tested and assessed, the trade product Glassclad 18 achieved the best results. One disadvantage of these glass modifiers is their limited ability to be worked together with other, hydrophilous restoration materials. Tests showed that the ability of such mixtures to adhere to the surface of the glass was reduced.
The efficiency of protective layers that provide a barrier to diffusion was tested on glass by laminating it with thin perfluorated polymer foils. The problems of the poor adhesion of the glues used with the foils (both initial and longer-term), and the unattractive appearance of the glass/glue/foil combination could not be resolved in the limited range of the experiments undertaken for this study. The few samples that remained intact long enough after being subjected to the standard corrosion procedure confirmed that they offered satisfactory levels of protection, but because of the problems already mentioned one must conclude that it is not possible to produce a sufficiently durable protective layer in this way.
4. Practical Tests on the Consolidation of Paint Outlines: a Comparison of Various Conservation Materials
4.1. Outline of the Task
The stabilization of endangered paint layers is one of the central problems in glass conservation. Loose paint outlines that have been infiltrated by corrosion must be bound to the glass beneath if they are not to be eventually lost. Depending on the state of the stained glass, cleaning of the surface of the glass should be undertaken before this process is begun. It has already been noted that the acrylic resin Paraloid B72 has been used for the conservation of stained glass widely since the early 1980s, at least by studios in Germany. It can be dissolved in various solvents and can be applied in differing concentrations (see sections 2 and 3).
The Fraunhofer-Institute für Silicatforschung (the Fraunhofer Institute for Silicate Research, ISC) has developed two new types of material specially for the conservation of historic stained glass (Fuchs, Römich, Leissner and Tur 1991; Römich, Pilz and Fuchs 1993). One of these types consists of organically modified silicium formulations under the brand name ORMOCER, patented by the Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung, Munich (the Fraunhofer Society for the Promotion of Applied Research).
These products are also known as heteropolysiloxanes or hybrid polymers, as they occupy a middle ground, in terms of their structure and characeteristics, between organic and inorganic polymers. The ORMOCER products are therefore capable of forming stable bonds with inorganic underlying layers, such as glass, as well as organic ones. These products can also be used in combination with Paraloid B72 as a protective coating for glass that is sensitive to corrosion. For this purpose, tiny flakes of glass are added to the mixture to act as a diffusion barrier for vapour. Several pilot studies were set up in 1986 to test the effectiveness of this combination layer on original stained glass. ORMOCER products can be also used in diluted form in combination with organic polymers to consolidate paint layers. In the case of the Crodel Window in Erfurt, mixtures of ORMOCER and Paraloid B72 have already been applied over large areas (Römich, Pilz and Fuchs 1993).
The second new type of conservation material developed by the ISC is known as SZA. Silicium alkoxides and zirconium alkoxides are subjected in the laboratory to a hydrolysis-condensation process to produce a pre-condensed reactive product (SZA), which after application to paint layers combines with moisture in the air to form an inorganic gel (Römich, Pilz and Fuchs 1993). This inorganic SZA compound has the advantage that it can form stable bonds with glass; alcohol (iso-butanol) should be used as a solvent. After hardening, SZA is no longer soluble with organic solvents, and treatment with it is therefore irreversible; this means of course that the application of this substance should be confined to the exact areas where the paint layers require consolidation. The advantage here is that SZA does not block the pores of the paint layers, so these can be treated subsequently with other materials (for example Paraloid) without having to remove the SZA first. Since 1990, there have been several pilot studies using SZA, mostly on nineteenth-century stained glass. Assessment of the results of these tests in the near future will show if SZA has a future as a conservation material for routine use.
Of course, in stained glass conservation it is not just the durability of newly developed materials that is important, but how easy it is to work with them in practice, and any advantages they may have compared with products already in use. For this reason the glass restoration workshop of the Cologne Cathedral fabric works has been carrying out experiments to compare the use of ORMOCER and SZA in practice with Paraloid B72, and to test if Paraloid B72 can be optimized as a paint stabilization agent, for example by varying the solvents used and their concentration.
4.2. The Test Process
In order to simulate real conditions, the tests were carried out on modern antique glass with artificially corroded paint layers. Damaged contours can be simulated by carefully varying the usual processes involved in mixing the stained glass paint. Normally stained glass paint in powder form is mixed with a binder (gum arabic, dammar-based lacquer, etc.) and then worked with a medium such as water or turpentine. If the proportion of binding agent is raised disproportionately, the paint outlines can tear when drying out or during firing, or can flake away in clumps from the base layer. Different patterns of damage can be produced, depending on the amount of bonding agent added to the mixture.
Sheets of modern antique glass measuring approximately 7cm by 15cm were specially made for these tests. The paint colours were mixed with disproportionately large amounts of gum arabic, and the medium used was vinegar. Cracks and damage in the paintwork were already apparent during drying, and developed further during firing. Paint outlines are generally fired at 600°C, but these test sheets were fired at lower temperatures, and included dummies fired at 300°C. This produced paint outlines that adhered only loosely to the surface contours, and which lifted off in clumps. When less gum arabic was added, porous and powdery paint outlines were produced. Other dummies were fired at 540°C; this produced poorly fired paint outlines that were not completely fused to the glass beneath. They seemed 'boiled' in appearance, and patterns of cracks appeared in certain areas. In this way, it was possible to simulate the normal paint damage, by using different bonding agents and varying the firing temperature.
The materials tested were applied to the artificially damaged paint outlines, and a small area of glass was left untreated for comparison (fig. 8). Two weeks after the application, the adhesion of the contours to the glass was tested with self-adhesive transparent foil, which was applied to both the treated and untreated areas, gently rubbed on with the thumb, then immediately pulled off and stuck to white paper. This gave a good idea of the consolidating effect of the various materials. While with most of the untreated paint outlines all of the paint stuck to the foil, the treated contours did so only partially. Consolidation ability could thus be measured by the amount of paint contour removed.
The following questions were of prime importance in this series of tests.
- How can the materials be worked?
- What degree of fluidity is appropriate?
- How frequently must the materials be applied in order to achieve a functional level of consolidation?
- How did the surface of the paint layers change during treatment?
4.3. Test A: Comparison of Various Materials
In the first test series the newly developed ORMOCER and SZA products were compared with Paraloid B72 (fig. 9).
Paraloid B72
In this test, Paraloid B72 was applied in an ethyl-acetate solution, with 10% Paraloid B72; this relatively high percentage was chosen deliberately. At this concentration Paraloid B72 only needs to be applied once in order to stabilize paint outlines effectively. The degree of stability achieved in this way served as a benchmark the other materials. Whether this level of stability is necessary for original glass is not under discussion here.
Paraloid B72 can be used by any conservator, is easy to handle, and can be applied is very specific places. It has the advantage that its viscosity can be varied without reducing its effectiveness. In this way, loose flakes of stained glass paint can be glued with an extremely viscous solution, and poorly fired paint soaked in a more fluid solution; this issue was particularly addressed in the second test series. However, paint outlines that have been treated with Paraloid B72 tend to become shiny and visibly darker. Treatment with Paraloid can therefore always be observed, depending on way in which the light falls.
Paraloid is considered to be chemically very stable (see section 2). However, it is a purely organic material, and contact with the inorganic surface of the glass could mask risks under specific circumstances (for example, flaking of the polymer film or paintwork as the conservation material ages).
ORMOCER
For this test series, a mixture was used, 80% of which consisted of ORMOCER, 10% Paraloid B72, and 10% the acrylic resin Plexigum N80 (produced by the firm Röhm of Darmstadt); this was dissolved in ethyl acetate (6% solution) (Römich, Pilz and Fuchs 1993). In practice, ORMOCER behaves similarly to a solution with a low concentration of Paraloid: it flows easily into the paint outlines, but occasionally also over the edges of the outlines. With dirty or corroded glass surfaces, this can lead to the consolidation of areas that should in fact not be consolidated.
ORMOCER mixtures have to be applied several times to achieve optimal stabilization, with an interval of at least 24 hours between each application. The paint outlines become more and more solids from application to application. Increasing saturation of the paint outlines was apparent, though bonding to the surface of the glass was not: this adhesion only comes into effect days after the final application. This treatment leads to a slight change in the visual impression given by the surface of the glass, because the paint outlines darken a little.
SZA
SZA was applied as a 14% solution in iso-butanol (Römich, Pilz and Fuchs 1993). SZA is not intended to glue the paint-layer particles together, rather it consolidates the paintwork's porous structure, which then has to be reattached to the surface of the glass. For localized application, SZA is difficult to handle, as it is extremely fluid: it and runs very well into the paint outlines, but also far beyond the edge of the paint outline, if the brush used is overwetted.
SZA is positively soaked up by raw, powdery paintwork. Since its possesses no adhesive qualities, it is of no use for fixing paintwork lying like shells on the surface of the glass. For optimum effect, SZA should be applied several times, allowing a gap of several days between applications for it to set. During this time, the humidity in the workshop needs to be measured carefully, as SZA requires at least 50% humidity to set properly. SZA can be stored for a maximum of only six weeks.
Even after repeated application, SZA does not change the appearance of the paint outlines in reflected light. This means that it is more suitable for the fixing of coatings or even application over wide areas than any other material consolidant. Since SZA combines to form an inorganic gel, the danger of long-term damage is less than with organic polymers.
Fluidity
For this test, a drop of each consolidant was dropped onto a dummy to observe the way in which it soaked into the paint layers. The best absorption into the paint was clearly that demonstrated by SZA.
Adhesive Strength
To test the adhesive effects of these materials, two weeks after treatment, adhesive foil was applied to the paintwork and removed. The results can be seen in fig. 11. The paintwork of the untreated section was removed completely, but that in the treated areas held very well. The best results were achieved with Paraloid B72; with ORMOCER and SZA small amounts of pigment came off with the foil. Of course such a test is unrealistic, in that these materials would never face such extreme stress in practice; nevertheless the results effectively illustrate the adhesive strength of these agents. They also offer the possibility of identifying the optimal number of applications of each material to achieve maximum adhesion. In this present case, Paraloid B72 only had to be applied once in a 10% solution, whereas SZA had to be applied four times, and ORMOCER three times to achieve the same adhesion.
Summary of the Results
From a restoration point of view, Paraloid B72, SZA and ORMOCER are roughly equal in terms of their ability to stabilize endangered paintwork. The decision which to use depends on the nature of the damage to the paint outlines. Paraloid B72 is extremely flexible in use: it can be used equally well for stabilizing loose paint layers and flaking paint outlines.
ORMOCER has similar qualities to Paraloid B72, but in the mixtures used in the test does not possess the adhesive qualities of the former. This can be improved by increasing the proportion of Paraloid in the mixture. The inorganic composition of ORMOCER makes the material similar to glass, reducing the likelihood of long-term damage.
From a restoration point of view, SZA is particularly useful, since it effects practically no change to the surface of the glass; it is also inorganic and chemically very similar to glass. However, it is not suitable for all types of damage (for example, fixing of paint layers that are already flaking away from the glass beneath). It is ideal for fixing poorly fired paint layers and washes, and could also be used application over a wide area of painted surface.
4.4. Test B: Optimizing Paraloid B72
A second series of tests was aimed at exploring the solution formulas and concentrations in which Paraloid B72 can be best employed. To see if it developed different material qualities when dissolved in different solvents, Paraloid B72 was dissolved in ethyl acetate; toluene; a mixture of methyl ethyl ketone and ethanol; and a mixture of toluene and white spirit. These solvents were used in various concentrations of between 5% and 10% to test if this had any influence on its workability. Plextol B500 was used as an alternative to Paraloid B72 in the tests; this is a water-based dispersion of a polyethyl acrylate by the firm Röhm.
As expected, the lower concentrations dispersed more easily into the paint outlines. Of course such low concentrations also have less adhesive strength, but this can be remedied by repeated application. The tests with self-adhesive foil showed that a solution with 5% Paraloid B72 needed to be applied three times in order to achieve the same adhesive strength as a single application of a 10% solution. Nevertheless application of low-concentration solutions has its advantages: high concentrations can dry out prematurely on the glass or even on the brush, and if the material cannot penetrate to the base layer, no adhesion will be achieved. The lower the concentration, the less visible the Paraloid is in reflected light, an effect that diminishes however with repeated application.
Tests with various solvents and solvent mixtures showed that the commonly used toluene is most suitable. Ethyl acetate evaporates very quickly, with the resultant risk that the Paraloid B72 may not be sufficiently well dispersed into the paint outlines. The solvent mixtures did not evaporate so quickly, though they were in no way superior in terms of their application to toluene, and they further require the conservator to prepare and apply the mixture. Toluene is more toxic than the other materials and therefore a potential health risk; however, if the correct procedures are followed, its use should present no danger to the conservator.
The water-based acrylic-resin dispersion B500 proved to be surprisingly easy to work with for the stabilization of paint outlines, and was more workable in every respect than Paraloid B72. However, despite frequent applications it was not capable of fulfilling its core function: under the microscope it was apparent that the dispersion had not penetrated the contours; the reason for this was probably the high viscosity of the dispersion and the comparatively macro-molecular structure of the acrylic resin.
4.5. Summary
Both series of test showed how important it is to test new materials before using them on original glass - not just for the physical qualities that can be tested by technical criteria in the laboratory, but also for their practical application in conservation. Dummies are an excellent way of achieving this, allowing the ease of application and the effectiveness of these formulas to be tested.
Conservators should familiarize themselves with different materials for use in different cases, in order to be able to explore their potentials and their limits in practice. As was mentioned in previous sections, selecting the correct application method is also extremely important. The choice of solvent and concentration must be tailored to each particular case. It can happen that there are different degrees of damage to the paintwork within a single panel, each requiring different treatment. For example, shells of paint that are flaking off must be stabilized with more concentrated Paraloid solutions, while thinner solutions are preferable for stabilizing unstable but still adhering layers of paint.
If too little conservation agent is applied to the paint contours then the intervention will not have been carried out in an optimal manner. However, high-concentration solutions are often used in conservation practice, for example if containers are left open and solvents evaporate. The risks posed to stained glass can be considerable, as loose, poorly attached paint outlines covered with a thick layer of polymer can easily come loose, which can lead in certain circumstances to the complete loss of the paintwork.
5. Conclusions
All conservation and restoration - of stained glass as other media - must today be carried out in accordance with the primary commandment of minimal interference and maximum preservation of original material. The aim is no longer to restore objects to their original glory. It is rather a question of protecting as much as possible of the substance of often corroded or otherwise damaged ancient works of art from further deterioration. Any restoration of stained glass by replacing individual pieces, completing panels or retouching the paintwork of historical glass should, according to current understanding, only take place in exceptional circumstances, after thorough and careful justification. Indeed, restoration is by definition an exceptional measure, and should always take second place to conservation in the strictest sense of the word. The logical consequence of these basic principles for the conservation of stained glass (i.e., the fixing of breaks, the stabilization of loose paint layers, and the application of coatings to the surface of the glass to prevent corrosion) is the development and use modern techniques and materials. In this respect, modern conservation practice is committed almost exclusively to synthetic materials, usually artificial resins.
The research presented here on conservation and restoration materials constituted addressed in its first part the testing of natural and organic bonding agents used for the preservation of stained glass. Two approaches were described. The first was the testing of materials used in the past that had aged under natural conditions over many years, that is they had been exposed to the effects of the climate and the environment. The characterization of these synthetic resins by analysis, the determination of what changes and damage had occurred, and the testing of their overall effectiveness yielded important information about their durability.
Furthermore, these studies yielded insights into the history of conservation. They showed clearly how synthetic products developed for industrial or technical purposes were used in the past by stained glass conservators, often without modification. The disadvantageous effects that came to light in the course of this research could in the main be attributed to the fact that these materials could not fulfil all the requirements made of them on account of their chemical composition and physical make-up. This is true of the glues used for edge-bonding that fell into the category of dual-component resins (their firmness when set and adhesive powers were often far too great), as well as of the acrylic resins used for the consolidation of paint layers of the coating of surfaces (which demonstrated a high degree of durability, but proved incapable of forming a sufficiently lasting and permanent bond with the glass beneath). The studies also showed that the problems associated with these materials were often caused by applying the substances too thickly or too widely, rather than by any deficiencies in the bonding agents themselves.
In general the studies clearly showed that the majority of the organic bonding agents commonly used in the past was only suitable within certain parameters, as many provided clear evidence of changes following relatively short exposure to the elements, particularly those applied to glass exposed to the outside elements. The main reason for this is the vulnerability of the materials to the reactions of the ageing process, particularly in the presence of light and oxygen; in addition, these materials possess different chemical properties to glass. Most important here are their thermal and moisture-related qualities, which can often lead to tensions building up within the film. For these reasons, the most important preventative measure that should be considered when planning the use of such conservation materials is the installation of protective glazing.
Section 2 presented the results of experiments on synthetic conservation materials, which were tested under laboratory conditions for their ageing properties and long-term stability. Selected polymer-based materials used for edge-bonding, paint-layer consolidation and the conservation of stained glass were subjected to a standard corrosion procedure, and research was also conducted into materials in common use (the studio samples) as alternatives.
Among the restoration materials commonly in use in the workshop, epoxy resins, ultra-violet-reactive acrylic resins, silicon rubbers, and so-called instant glues were tested for their potential as edge-bonding adhesives. In practice, epoxy resins are the substances most commonly used for this purpose, because of their adhesive strength and durability. However, all the products in this category yellowed to a greater or lesser extent when subjected to ultra-violet radiation, although such yellowing is only a small disadvantage with narrow cracks. Ultra-violet-reactive resins also exhibited varying degrees of yellowing; additives such as activators can exacerbate this tendency. Silicon rubbers proved stable under simulated climate conditions, but their visual and physical qualities make them unsuitable as break-fixers, because they are highly viscous, soft, and cloudy; their inability to be redissolved is a further disadvantage when considering their use on historical glass. Instant glues, mostly cyanacrylates, do not yellow, but their adhesion to glass is very unsatisfactory.
Materials commonly used in workshops as paint-layer consolidants, included waxes, acrylic resins and some silicium-organic products, were tested. The behaviour of the waxes varied according to the temperature: at low temperatures they tended to be brittle and prone to crack, at higher temperatures they softened and were capable of expanding to fill the gaps. They are opaque and not colourless, but showed no tendency to yellow in the tests. The excellent ability of the widely used polyacrylate Paraloid B72 to withstand weathering was confirmed by the tests, though its ability to adhere to the glass surface was considerably reduced in humid weathering conditions.
Apart from the materials commonly used in workshop practice, alternative types of edge-bonding agents were also tested in the laboratory; this confirmed the general material qualities of these product groups. In a special series of tests, the viscosity of the thin-fluid epoxy glues was increased by adding highly dispersive silica gel, to see if this made them easier to apply. The durability tests showed a slight reduction in the glues' adhesive abilities with higher concentrations of additive. Some of the ultra-violet-reactive glues proved to have the best results overall. The newly developed light-reactive glues proved not to possess the necessary adhesive and cohesive qualities to fix glass breaks satisfactorily. Silicon rubbers generally proved to be durable adhesives, but were on various grounds unsuitable for break fixing.
The tests on alternative materials developed for paint-layer consolidation were the most comprehensive, and the following products were analyzed: stone-conservation materials based on polysiloxanes and polyurethanes; clear lacquers based on polyurethanes and alkides; and artificial resins based on acrylic and epoxy resins. In addition, aminosilane adhesion facilitators were added to improve the adhesive durability of these substances. The addition of polysiloxanes to these formulations and the coating of glass with polysiloxanes were intended to improve the hydrophobic qualities of the artificial resins.
The results clearly showed that of the materials tested only the products that fell into the categories of polyurethane lacquers and acrylic resins were suitable for paint-layer consolidation. Balancing up the test results and further advantages and disadvantages of these products (such as the ease with which they could be handled, their reversibility, and their behaviour in storage), it was found that formulations composed of a polyacrylate or polyurethane combined with an adhesive agent (with or without hydrophobizing components) achieved the best results; polyurethane formulations also came out on top when considering visual effect, adhesive ability, durability, and the level of protection afforded by the coating. However, the irreversibility of these interlinking, dual-component polyurethanes is a big disadvantage. Polyacrylates on the other hand, even those modified with polysiloxanes, are soluble with organic solvents.
Before these substances are introduced into restoration practice, they must all be tested in pilot studies under studio conditions and after natural weathering, in order to evaluate their ability to stand as useful alternatives to the most commonly used paint-layer consolidant Paraloid B72. The simulated environmental conditions of the laboratory are useful for comparing the various substances with each other, but they are not directly transferable to the weathering process in real terms.
Materials used for the conservation of corrosion-sensitive glass can be divided into three main categories: thick, lacquer-like layers; thin hydrophobizing films; and diffusion barriers in foil form. These conservation coatings were for the most part subjected to the same experiment and testing procedures as the paint-layer consolidants described above.
None of the substances tested proved to provide 'ideal' protection. To name but a few disadvantages, lacquer-like layers tended to peel away, thin hydrophobizing films are irreversible, and foils are visually intrusive. Since the application of such agents to the entire surface can have negative consequences, undertaking such measures without another form of protection such as external glazing is not advisable.
Section 4 described the results of a series of tests on various materials used to consolidate paint outlines. The first series compared the newly developed ORMOCER and SZA stabilization products with the tried and tested acrylic-resin Paraloid B72. The results showed that all three substances are suitable for stabilizing endangered paintwork, but varied in their consolidating qualities. While SZA is suitable for fixing poorly fired contours, coatings and paint washes, even over large areas, ORMOCER and Paraloid B72 were better at fixing loose flakes of paint. The decision as to which product to use therefore depends on the type of damage to be rectified. A second series of test focussed on optimizing Paraloid B72 for the practical applications as a paint-outline consolidant of contours. For this the types of solvents were varied, as were the concentration of the solids and the viscosity of the solution.
Both series of tests showed how important it is to test new materials before they are used on original glass, not just in laboratory conditions according to technical criteria, but also from a restoration point of view. The ideal test objects are sample sheets of real antique glass with artificially damaged paintwork. These dummies allow the ease with which new materials can be handled to be thoroughly tested and optimized, and their effectiveness assessed easily and in a manner appropriate to workshop practice. Conservators should familiarize themselves with different materials in order to be able to assess their potentials and limits in practice.
The use of artificial resins for stained glass conservation is problematic and can constitute a danger in itself, as the results of the tests show; nevertheless they are still very much in use in modern conservation practice, as no realistic alternatives have been found. The decisive factor for the success of a conservation treatment is the correct choice of materials, which must be durable and appropriate for the specific form of damage. The results of the systematic laboratory tests conducted in the context of this research project should prove helpful. Perhaps even more important. however. is the correct application of materials, which must be tailored for each individual case.
1 While the make-up of inorganic materials such as glass, glass corrosion products, metals and metallic compounds can usually be analyzed accurately either by determining their constituent elements (by X-ray fluorescence spectroscopy) or their crystalline structure (by X-ray diffractometry), the principal methods used for analysing organic compounds and determining their intra- and extra-molecular structures are spectroscopic, e.g., mass spectroscopy, ultra-violet/visible spectroscopy, infra-red spectroscopy, and nuclear magnetic resonance spectroscopy. Chromatography also plays an important role in determining chemical make-up and characteristics. For a more detailed description of the analytical methods used in the context of the research out lined here, as well as data sets from control experiments undertaken as preliminaries to the experiments on samples outlined here, see the final report on this research (Jägers and Brinkmann 1999).
2 Typically one needs to establish the following about samples of surface deposit: the constituents of the glass paint used for the original work and that used for any subsequent repainting or retouching; the constituents of dirt deposits; possible conservation agents; and the potential for damage caused by micro-organisms. Very different types of analysis are needed to gather this information. Where a sample is very small, such a complex series of questions can only be answered successfully if all the data concerning the object, its history, its make-up and condition can be taken into consideration.
3 The most important producers in Germany are Hoechst (product name: Mowilith) and Wacker (product name: Vinnapas). In addition, several firms have polyvinyl acetates in ready-manufactured form on offer. Products that are common in the trade include Uhu universal glue, Ponal, Bindan, etc (all polyvinyl-acetate dispersions); see further the manufacuturers' product information.
4 The temperature at which a compound melts to form glass is an important measurable characteristic of materials that have an amorphous glass-like structure. It is the temperature at which a brittle, glass-like substance changes into an elastic state, and is a way of measuring that substance's physical characteristics. If the melting point is low (e.g., -8°C), then the substances is very soft and sticky at room temperature; if the substance has a high melting point (e.g., 80°C), then it is non-sticky and solid. See further Schilling 1989.
5 The most important producers in Germany are Röhm in Darmstadt (product names: Plexigum, Plexiglas, Plexisol, Plextol); Rohm & Hass (product names: Paraloid, Primal); and BASF (product name: Acronal).
6 Produced by Hoechst (product name: Hostacoll C).
7 Various Ciba-Geigy products.
8 Produced by Glasbau Hahn in Frankfurt am Main (product name: SH1).
9 Produced by Disbon in Ober-Ramstadt (product name: Disbon-Glaskleber).
10 Produced by Herbert Hillary in Austin, USA (product name: Hxtal-Nyl-1)
11 Produced by Akemi in Nuremberg (product name: Akemi).
12 Produced by Wacker.
13 Internal report by P. Berkenkopf (Anwendung und Untersuchung bezüglich der Sicherung von Schwarzlotkonturen mit Silicon BS31) produced for the Cologne Cathedral workshop in October 1992.
14 A brand of the Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., in Munich.
6. Acknowledgements
Authors: Elisabeth Jägers, Hannelore Römich, Carola Müller-Weinitsche. This text originally appeared as chapter 6, 'Konservierungsmaterialien und Methoden' (pp. 129–66), in A. Wolff (ed.), Restaurierung und Konservierung historischer Glasmalereien, Mainz, 2000.
Publisher: Verlag Philipp von Zabern.
Translators: Joseph Elders, Joseph Spooner, Sebastian Strobl
Images: Bayerische Hofglasmalerei G. van Treck, Munich (fig. 1); Dombauhütte Köln, Glaswerkstatt (figs 2–4, 8–11); H. Marschner, Bayerisches Landesamt für Denkmalpflege, Munich (figs 5–7).