Corpus Vitrearum Medii Aevi
Medieval Stained Glass in Great Britain
Your trail:
- Conservation
- Science in the Service of Restoration
Science in the Service of Restoration
(Wolfgang Müller, Manfred Torge, Karin Adam, Hannelore Römich, Rudolf Weissmann, Rainer Drewello)
1. Questions Posed and Research Methodology
Stained glass is a particularly sensitive medium that in many churches has still not received sufficient protection against destructive influences of many and various kinds. Over long periods of time, nearly all materials are subject to chemical and physical change. The dramatic scale on which the processes of decay often occur results from the influence of corrosive gases that are part of the mix of today’s atmosphere. A basic requirement in the preservation of any historical monument is therefore interdisciplinary collaboration; only the joint efforts of art historians, restorers and technicians, as well as scientists, offer a chance to protect our artistic and cultural heritage from decline.
Measures to protect, conserve and restore stained glass are carried out by glass studios. The project on which this chapter reports provides support for this practical work through scientific back-up. In order to clarify what are very complex issues, a brief explanation of the scientific/technical context follows here, to preface the exposition of the basic questions posed.
1.1 The Peculiarities of the Materials
Stained glass consists of three basic materials: coloured, hand-blown sheet glass; the substance with which the painting is executed (glass paint); and lead. In the central and northern lands of Europe in the middle ages, glass was produced by melting sand and plant ash together; other additives were different types of lime, as well as, above all, metal oxides, used to colour the glass. The melting and glass-working techniques in the forest workshops required relatively high proportions of ash and lime to be added, with the result that the chemical compositions of these glasses are substantially different from those of glasses made by modern production methods. The proportions of the components naturally vary greatly from period to period and place to place, but also depend on the type of glass concerned. Its susceptibility to mechanical and above all hydrolitic damage arises from its low SiO2 content (on average 45–55% by mass). The effects of these types of damage are exacerbated by faults in the production of the glass, such as inhomogeneous areas resulting from blowing or mixing errors, which are nevertheless desirable in some respects, on account of the optical effect they produce. Glass with a content of c.15–25% by mass of each of K2O and CaO provides little resistance to corrosive decomposition of the glass surface; the leaching of alkalis and alkaline earth metals from the upper layers begins soon after the glass has been produced, and leads, sometimes within weeks, to SiO2-rich, water-retaining gel zones a few thousandths of a millimetre thick, which can be detected through their slight irridescence, and later through the dulling of the surface they cause.
Over the course of centuries, the gel layers have increased in size; the components that have leached out have reacted with acids in the environment, and the reaction products (generally gypsum and syngenite), mixed with dirt deposits, now form crusts that are very light-absorbent, especially on the outer surface of the window. It is difficult to assess the significance of these crusts in the further decomposition of the glass; in addition to detracting from the visual impact, there is the undoubtedly also the issue of their acting as a moisture reservoir. Removal of this so-called Wetterstein (‘weather stone’, weathering crust) on conservation grounds would therefore seem desirable. On the other hand, since the two zones – that is, both the gel layer, with its reduced levels of alkalis and alkaline earth metals, and the crust of corrosion products – protect the unaltered core glass, the composition of which remains unchanged, against the environment, crusts offer effective protection against continued hydrolitic decomposition of the glass. One of the most important aspects of the project was therefore the scientific evaluation of cleaning methods.
1.2 The Influence of Chemical Compositions on the Characteristics of Glass
Sand and plant ash do not only provide the main components of medieval glasses. In addition to several elements introduced in lower concentrations (Al, P, Mg), which generally have a favourable influence on the durability of the glass, colouring agents such as iron and manganese ended up in the mixtures through trace elements and impurities in the sand and ash. Depending on the oxygen partial pressure in the atmosphere in which the melting took place, a colour palette ranging from green through brownish tones to violet could be achieved, without having to use further colouring additives (Sellner, Oel and Camara 1979). On the other hand, copper oxide was certainly consciously used as a colouring additive, because red glass cannot be produced in any other way. All red glasses found among stained glass of the medieval period are Kupferrubine (‘copper rubies’), which were essentially manufactured as flashed glass, that is, with a thin layer of red glass on a colourless base glass. Glass colour is one of the defining aspects of stained glass as an art form, yet the colouring oxides present in the material, on account of their low concentrations, are of practically no significance for the chemical durability of the glass and therefore for any conservation measures.
Medieval glass paint shares the chemically unstable properties of the glasses. It often weathered as a result of its porosity, because moisture remained trapped in the material for longer; in addition it changed chemically as a result of contact with water and the elements. Thus in many cases such contour lines as are still present consist only of corrosion products, and any thin washes, even those on the internal face of the window, have already disappeared long ago through weathering. Generally speaking, painting in vitreous paint on the external faces of windows is no longer detectable. All medieval stained glass suffers from ever-increasing losses in glass-paint adhesion, though the degree to which this happens varies considerably. Even relatively intact trace-line layers are attacked from the side and from beneath on account of the decomposition of the glass surface beneath, adhering still only at a very small number of points. In such cases the restorer must undertake consolidation. Because the glass cannot be warmed to the temperature at which the glass-paint would soften, or even near it (because of the condition of the glass and the threat of possible changes in colour), consolidation as an emergency measure has to be effected using artificial resins or waxes. The fixing of glass-paint is a very difficult job and was the object of considerable research during the course of the project’s work.
Nineteenth-century stained glass presents problems that are essentially very different from those associated with medieval glass. The glasses have chemical compositions corresponding to those of glass used in modern buildings. With contents of 70% by mass of SiO2, 12–16% by mass of Na2O, and 6–12% by mass of CaO, they are far more resistant to the effects of weathering. Vitreous paints and layers of coloured enamel in nineteenth-century stained glass often exhibit high levels of damage from corrosion however; in addition, it should be noted that in many cases, these windows, which – unlike their medieval counterparts – were not usually removed to safe storage during the war, often contain gaps that have to be filled by the restorer.
All measures undertaken for the protection, restoration and conservation of historical stained glass are geared towards exerting an influence on the slow but continual processes of change in the material. In the end, these processes, the speed of which can be changed through the interventions, determine the effectiveness of the preservation measures. Therefore, a theoretical investigation into the many individual problems is desirable. But since the processes and their mechanisms are very complex, and because the resources available for this project had to be directed above all towards answering practical questions, the possibilities for this were very limited.
1.3 The Project’s Areas of Investigation
Two essential documents form a framework for works carried out on stained glass in the context of care for historical monuments. At the XV. International Colloquium of the Corpus Vitrearum in Amsterdam, the ‘Guidelines for the Conservation of Historical Monumental Stained Glass’ were conceived (CVMA and ICOMOS 1989). An updated version followed in 1993 through the work of the national committee of the German Corpus Vitrearum Medii Aevi (ICOMOS 1993). [Translators’ note. The most recent version of these guidelines, drawn up at the Nuremberg colloquium in 2004, is available here].
Every restoration studio that deals with historical objects has general requirements to conform to. These preconditions are not only binding for the restorer, they dictate similarly the goals of scientific/technical research, towards which all treatment strategies should be directed.
- the greatest possible retention of the original substance
- the highest possible protection against expected future damage (conservation)
- the closest possible rendition of the original artistic appearance (restoration)
- consideration of the possibility of undoing any interventions applied (reversibility)
- exact description of the state of the original and recording of the interventions applied (documentation)
All partners involved in the carrying out of this project had to commit themselves to fulfilling these requirements. Overall, the many different individual tasks were so varied, that what follows can only be a summary of nexuses of tasks. Thereafter, results of fundamental importance are given by way of examples. As far as extensive presentation of the research and its results are concerned, the reader should consult the final reports of the individual project partners. It was planned that tasks should be assigned to groups of problems as follows.
- characterization of the material, damage analysis, research into the causes of damage (Bundesanstalt für Materialforschung, Berlin [BAM]; Institut für Werkstoffwissenschaften, Erlangen [IWW]; Bayerisches Landesamt für Denkmalpflege, Munich [BLD]; van Treeck studio, Munich)
- development and modification of methods for the protection, conservation and restoration of glass, with reference to cleaning (Fachhochschule, Cologne; Fraunhofer Institut für Silicatforschung, Würzburg [ISF]; BAM), the treatment of browning (BAM; IWW), coatings and consolidants (ISF; BLD), and the optimization of external protective glazing (Oidtmann studio, Linnich; ISF)
- establishment of concepts for objects, guidelines and recommendations, object monitoring (all project partners)
For the consolidation and conservation of glass in the studios very different methods – the damage and protection potential of which were to be researched more closely, both in general and in concrete individual cases – were applied to two problems: the removal of layers of corrosion products (cleaning, exposure of the glass surface and/or of paint layers) and the fixing of loose glass-paint. Following on from this, it was deemed necessary to develop a reliable research method with which mechanical types of cleaning method of different intensities could be assessed. Furthermore, alternatives for the removal or reduction of these layers, or the modification of their visual effect, had to be tested. In the particular case of so-called ‘browning’ – the powerful absorption of light effected by manganese compounds in the gel layers – it was thought necessary to undertake more detailed research with the help of model glasses, using the method of browning reduction involving hydrazine hydrate solutions already trialled.
1.4 Research Methods
The principal area of concern for research into the preservation of stained glass was the translation of the general guidelines of the international CVMA committee into concrete, practical measures that could be used by the restorer. Where possible, reliable methods (with which, most importantly, the damage potential of cleaning measures can be evaluated) should be employed in addition to what has been the usual practice until now, of assessing the damage status visually, albeit slightly magnified with a magnifying glass or stereomicroscope. Photographs of details of the surface taken at microscopic level, particularly of paint layers, as well as in special cases electron-microscope images of prepared sections perpendicular to the surface, have long proved their worth in determining the measures necessary for securing paint layers or cleaning. Magnification of x200 can be achieved with a microscope, and of approximately x2000 with an electron microscope, and this reveals many details in the structure of the surface that remain undetected by the naked eye.
On the other hand, these methods were geared towards use on specific objects, as it is hardly possible to make generalized recommendations. Every individual case exhibits specific phenomena that require treatments directed towards these particular features. It is therefore also necessary that the questions asked by restorers are focused on specific ends and posed accurately; to answer them often requires the adoption of several different research methods. Practically no concrete example can be solved with a single swift measurement.
In addition to the general issues addressed so far, specific questions concerning materials frequently arise as a result of previous restorations. Instances of cold painting and coatings of a whole variety of varnishes, applied either as a protective measure or to modify the visual effect (e.g., cellulose lacquer, emulsion paints, varnish, etc.), as well as remnants of putties and glues, have to be identified. Following on from this, ways to remove them must be found that afford the highest possible level of protection while being commercially viable. Appropriate research undertaken to very different degrees was planned for the individual objects, and on each occasion was intended to result in recommendations that could be included in the research reports in written form as well as being translated into practice through direct contact with studios and bodies responsible for the care of historical monuments. Complementary scientific support of restoration works must not only be adopted to oversee all necessary investigations, it must also ensure that the execution of work in practice is in line with the most recent scientific knowledge. A particular concern for the project was to promote mutual understanding between the disciplines and find a common language.
The variety of possible research methods employable is virtually limitless, if in addition to measurements taken on original materials one includes measurements made on model glasses produced in imitation of original ones, which can then be used as the working material and exposed, for example, to accelerated weathering, or destroyed in the course of measuring processes. On the other hand, all investigations into original material must be carried out in a non-destructive manner; if need be, a negligible loss of original material can be accepted, as happens for example when a section is created on the edge of a sample of original glass. The fact that a few tenths of a millimetre of the glass have been removed remains hidden beneath the surrounding lead profile. Of the suitable, non-destructive measurement and research methods, or at least those requiring only the very smallest amounts of sample, the following were used within the context of the project: light microscopy; electron microscopy; energy- or rather wavelength-dispersive X-ray spectroscopy with an electron microprobe; X-ray diffractometry; X-ray fluorescence analysis; infrared spectroscopy; ultraviolet spectroscopy; surface profile measurements; and microbiological procedures. Nearly all investigations were carried out either on surface layers that had been removed (corrosion products), on undecayed surfaces, or sections that were perpendicular to the surface; the latter took place exclusively on the edges of samples or on the surfaces of breaks, with the loss of material reduced to a minimum.
These investigations allow us to gain an insight into the structure and morphology of the surface layers, and are therefore indispensable, for example, when assessing cleaning interventions. At the same time, they permit us to determine in a non-destructive manner the composition of the gel layer and core glass with the aid of an electron microprobe. The samples must be sufficiently stable however that they can tolerate being held temporarily in the vacuum of the measuring chamber.
Even more extensive were the research methods applied to model glasses, which were subjected to deliberate damage through treatments of very different kinds, from being exposed to high levels of corrosive gases and moisture in the climate chamber to being stored directly in water or acid, from deliberate mechanical damage to testing on occasion to destruction. To aid comprehension, necessary details of research methods are reported in the descriptions of individual examples.
2. Research on Original Samples
2.1 The Chemical Compositions of Medieval Glasses
Practically no historical window has retained its original appearance for the modern viewer. Moisture and corrosive substances in the air attack both the glass and the vitreous paint applied to it. The glass is corroded hydrolitically.
The extent to which the corrosion takes hold is essentially governed by the chemical composition of the glasses. At the start of the project, the results of the analyses of c.300–400 samples of medieval glass of very different provenances were known; a division into five characteristic types had already been undertaken (Müller 1992). Apart from some insignificant exceptions, with medieval glass we are dealing with the least durable types of silicate glass (1 and 2), those containing potassium and calcium (see Table 1). Glasses of type 1 have a ratio of CaO to K2O of 1:1; the range lies between 1:2 and 17:10 and is consistent with the addition of plant ash as a raw material. Type 2 brings together glasses that exhibit a ratio of CaO to K2O of >2:1. This calcium-rich type can be traced back to a glass produced from plant ash, lime and sand.
In type 5 we find roughly equal quantities of K2O and Na2O, which when taken together do not exceed the amount of CaO. This glass type, which has a notably higher SiO2 content than the type 1 and 2 glasses, is characteristic of Renaissance panels of the fifteenth and sixteenth centuries. A type of blue glass (type 3), which is found only in the twelfth century, mainly in England and France, was identified as soda-lime glass, and in its composition closely resembles glasses produced in antiquity
There is furthermore an emerald-green type of glass, found throughout the middle ages with a wide geographical distribution, which is characterized by high levels of lead oxide (type 4). The division into glass types was accomplished without the aid of numerical methods, by through simple comparisons of the proportions of the various elements (Müller 1992). As a result, more than thirty different objects could be taken into consideration (including, among others, samples from Cologne and Erfurt cathedrals). The results were recorded, together with data derived from an evaluation of the relevant literature (Brill 1970; Cox and Gillies 1986; Gillies and Cox 1988; Schreiner 1988; Besborodov 1975), and assembled in a database (MIGLA), which on 20 March 1996 contained 619 analyses of glass.
| Type | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| SiO2 | 45–55 | 45–55 | 60–70 | 30–40 | 55–70 |
| CaO | 15–25 | 10–15 | 1–6 | 5–20 | 10–20 |
| K2O | 15–25 | 10–15 | 5–8 | 5–20 | 2–8 |
| Na2O | 0–2 | 0–2 | 10–20 | 0–1 | 2–8 |
| PbO | 0–1 | 0–1 | 0–1 | 10–50 | 0–1 |
| P2O5 | 0–4 | 0–4 | 0–4 | 0–10 | 3–10 |
| colour | variable | variable | blue | green | variable |
Deductions concerning the correlations between the composition of glass and state of corrosion are only possible if the state of weathering is also known. In about 60% of statements given in the literature, we find descriptions of the condition of glass that can be summarized in three categories: not weathered, pitted, and encrusted. Describing the condition of the glass surface in detail however is not useful, as judgements can be subjective. Data from more than thirty laboratories were included in the database. The analysis process thereby embraced the whole spectrum of procedures currently available for the analysis of glass: wet chemistry, flame photometry, X-ray diffractometry, and the electron microprobe. The MIGLA database was assembled with the help of statistics and graphics software (STAT-GRAPHICS, version 4.0). The glass analyses were ordered according to the dating of the glass by century and collected in files. Information is also retrievable on the provenances of the glasses, their colour, their state of weathering (as far as this was available), and source citations. For glasses only known from the specialist literature, determining provenance is often restricted to what is stated in the object specification. Only in the course of the project’s investigations was it possible to make precise statements in relation to the panel description, and sometimes even to document a piece of glass that had been analyzed within a panel. The colour was characterized by visual inspection, in line with its optical appearance. The database facilitated swift access to the results of analysis. On the basis of a glass’s chemical composition, it was possible to deduce prognoses regarding the future course of weathering. The classification of analysis data by century can also be used as a dating aid to determine the age of glasses of unknown provenance (e.g., excavated finds).
2.2 The Relationship between Chemical Composition and State of Corrosion in Medieval Glasses
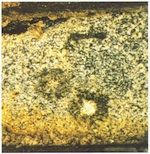
With reservations, the corrosion phenomena observed on medieval glasses can be associated with the individual glass types. Glasses of type 1 exhibit the severest corrosion damage. Generally, thick corrosion crusts are found on the external face (fig. 1), and the internal face of this glass type often exhibits a built-up uniform corrosion layer (fig. 2). Having said that, the extent of damage varies considerably from place to place, and clearly depends on the environmental conditions to which the glass has been exposed. The whole history of treatment during earlier restorations is also significant.
Because of their higher proportions of CaO and K2O, glasses of type 2 tend to be more resistant to corrosion than glasses of type 1. In favourable circumstances, they are often found to be only in the initial stages of corrosion (fig. 3). The glasses of the three remaining composition types clearly showed themselves to be more durable. As far as their quantities are concerned, they play practically no role in the preserved body of medieval stained glass: well over 90% of glasses belong to types 1 and 2.
The gel layers created as a result of the leaching of alkalis and earth alkalis from the surface layer of the glass consist of c.80–90% SiO2 and 10% water. What is known as Wetterstein builds up through the reaction of calcium and potassium ions leached from the glass with acid gases in the atmosphere (SO2, CO2), and consists primarily of sulphates CaSO4 • 2H2O (gypsum) and K2SO4 • CaSO4 • H2O (syngenite), as well as small quantities of calcite (CaCO3), quartz (SiO2), and amorphous silicic acid (fragments of the gel layer). Furthermore, darkly coloured materials can be deposited that have an even stronger light-absorbing effect, to the extent that one can speak of ‘browning’. The phenomenon can have very different causes, e.g., the deposit of melanins discussed in section 4 below, or the accumulation of manganese compounds dealt with briefly in the next section.
2.3 Manganese Browning
A typical form of browning damage – which mainly affects flesh-tone glass coloured with manganese, though in many medieval ensembles the entirety of the glazing is affected – has been known of for decades (Müller, Pouillon, Bochynek and Mehner 1986). It had been demonstrated long before the beginning of the project by means of electron microscopy and microanalytical research, that compounds of tri- and quadrivalent manganese, which accumulate in the corrosion product layers and particularly in the cracks in the gel layers, sometimes give rise to a total loss of transparency on account of their strong light-absorbing effect (Müller, Bochynek, Mittelstädt and Pouillon 1990; Fitz 1981). During the course of bench-scale testing, the possibility of improving transparency by reducing these compounds with the help of hydrazine to their manganese equivalents in the oxidation state +2 was also discovered to be one possible route in principle, and was tested on some examples in practice. Further experiments with original glass should however where possible be kept to a minimum. As a result, the work undertaken in the context of this project was carried out on model glasses produced to imitate original glass. These are described in section 3.2 below.
Browned glasses usually exhibit very high levels of manganese in the defective areas of the surface layers. In order to be able to make more detailed statements concerning their morphology, appropriate investigations were carried out on the medieval samples made available during the course of the project that exhibited any signs of browning. As an example, fig. 4 shows an electron-microscope photograph of a section through a gel layer with cracks, in which there are also crystalline deposits; fig. 5 shows the manganese distribution for the same test area. The correspondence between the two images demonstrates that in the glass sample suffering from browning there are considerable accumulations of manganese compounds in the surface layer. Similar manganese accumulations can be found in some layers of corrosion products. In those cases, a prerequisite for visible browning is the presence of fragments of the gel layer among the loose corrosion products. Glass of composition type 1 (see Table 1) in particular has a tendency to acquire this appearance in pertinent weathering conditions. Despite the large number of original objects investigated, no clear points of reference for browning were established, either in relation to glass composition or forms of corrosion, as both factors clearly play a role in influencing the situation. Surprisingly, an MnO content of only 0.3% by mass in the glass as a whole is enough to cause browning.
3. Model Glasses as Simulation Materials
The cultural heritage requirement that as much of any original material as possible be retained means that original glass is rarely available for most scientific research. Destructive investigations can be carried out on model materials. In any event, weathering conditions (which vary to an extraordinary extent over time and from place to place), as well as the variety of components in original glasses (together with the great variability of their proportions), allow only limited conclusions to be drawn concerning the relationships between the composition of glass and its state of decay. For this reason too, model glasses have long proved their worth in laboratory experiments (Newton 1982). [Translators’ note: this work, CVMA (GB), Occasional Paper II, is available here]. Important parameters that influence the corrosion of glass (e.g., method of production, treatment of the surface), can be applied in a targeted manner in order to produce samples comparable to original glasses for laboratory experiments. Unlike the originals, these samples can be used as the subjects of corrosion experiments, without having to restrict oneself to non-destructive methods of treatment and analysis.
In order to understand processes that extend over years on medieval glass within manageable periods of time for the purposes of research, the corrosion process can be accelerated in climate chambers. This is generally achieved by raising the relative moisture level in the chamber, or by implementing accelerated temperature/moisture cycles, as well as through the introduction of harmful substances. Simplified corrosion experiments can also be carried out by immersing glasses in acid.
In different laboratory experiments, it was possible to simulate cracked gel layers and built-up crusts (Marschner 1984; Müller 1997; Fuchs, Patzelt and Schmidt 1989). The range of patterns of damage achievable however in climate-chamber conditions does not cover the whole spectrum of phenomena observable on original glasses. For example, it was not possible to recreate the pitting typically found on medieval glass. It is even the case that corrosion processes that take place under accelerated weathering conditions in the climate chamber on model glasses of the same chemical composition as medieval glasses do not produce identical corrosion damage. The principal method of corrosion however can be simulated in the laboratory. In some cases, a time-consuming natural weathering was deliberately chosen, so that the state of corrosion of sensitive glasses under natural conditions could be characterized (Fuchs, Römich, Tur and Leissner 1991).
The ways in which model glasses can be used, for example in the assessment of conservation materials, have already been exploited in a variety of ways (Müller, Drachenberg and Pouillon 1980; Fuchs, Römich, Tur and Leissner 1991). The areas of application for model glasses within the context of this joint project are described in what follows. The advantages of using model glasses when assessing possible cleaning methods are set out in the chapter on cleaning. Simulated materials may be able to replace valuable original glasses in laboratory experiments, but one cannot assume that the results can be translated directly into practical action. Translatability must be discussed critically in each area of application.
3.1 Production Methods
Model glasses were deployed by different working groups within the context of this project. The choice of composition and method of production depended on the questions being posed in each case, and the technical facilities available in the laboratories. To a large extent, the Bundesanstalt für Materialforschung und -prüfung (BAM, The German Federal Authority for Researching and Testing Materials), based its choices of glass composition on the results of analysis of glasses of widely differing provenances. The model glasses were produced by melting together mixtures of laboratory chemical compounds. The glass was formed by pouring and rolling; sometimes the samples, which were about 2–5mm thick, were further worked by grinding and polishing. These glass samples were used for different experiments, some of which are described in section 3.2 below. At the Fraunhofer-Institute für Silicatforschung (The Fraunhofer Institute for Silicate Research), preference was given to potassium-rich potassium-lime-silicate glasses, which are especially sensitive to corrosion (Fuchs, Patzelt and Tünker 1989; Römich and Fuchs 1996; Fuchs, Römich, Tur and Leissner 1991). The glasses were melted in platinum crucibles and founded into blocks. Thin panes (2.5 x 3.0 x 0.7mm) could be obtained from these by means of a special saw, which had to work with paraffin cooling; their raw surfaces were smoothed by heating the samples to just above its transition temperature. This heat polishing is necessary to obtain consistently reproducible surfaces (Fuchs, Patzelt and Tünker 1989). One advantage to the institute’s model glasses lies in the fact that the corrosion process can be quantified by means of infrared spectroscopy; this means that these special glasses may be used as so-called glass sensors (see section 4 of the chapter on protective glazing).
At the Institut für Werkstoffwissenschaften (Institute for Material Science) at the University of Erlangen, model glasses were produced that could be used to research microorganisms on glass (see section 4 below). Their preparation was analogous to the method employed at the Bundesanstalt für Materialforschung und -prüfung.
At the Bayerisches Landesamt für Denkmalpflege (Bavarian Regional Authority for Care of Monuments) another production method was chosen, to produce a model glass that could be used to reproduce damage phenomena through corrosion experiments and to assess conservation materials (Marschner, Bertelmann and Tilenschi 1995; Marschner, Bertelmann, Striewski, Tilenschi, Koci and Schanz-Zepek 1996). Employing a process that is substantially similar to that used originally, the glass was manufactured at the Waldsassen studio by the cylinder glass-blowing technique.
3.2 Examples of Research
Knowledge concerning the causes of environmental damage to historical glass can be gleaned from the weathering of model glasses in climate chambers (Marschner 1984; Müller 1997; Römich 1998). In the context of this project however, model glasses were employed in the first place to optimize treatment measures (cleaning, conservation, fixing of glass-paint). The extensive preliminary experiments investigating the possible use of chemical cleaning methods that were carried out with close collaboration between the project partners are presented in the chapter on cleaning. Some of the experiments carried out at the BAM in Berlin, described below, which give an insight in the number of variety of possible uses for model glasses, should be seen as a complement to the issues outlined there.
Research into the Formation of Gel Layers and Crusts through Weathering Simulation
The simulation of weathering on different model glasses was carried out in a climate chamber equipped to deliver corrosive gases. In order to reproduce the damage processes in an accelerated manner, the corrosive gases NOx and SO2 were introduced into the climate chamber in a concentration about 300 times greater (5ppm) than is found in heavily polluted industrial areas. With a relatively high humidity of 85% the temperature was varied over a period of 96 hours between 40°C and 10°C (a day/night cycle), and subsequently kept at a constant -10°C for 65 hours (winter cycle).
Weekly examination at a microscopic level and analysis by electron-microprobe spectroscopy allowed the formation and development of the gel and corrosion-product layers to be observed. The extent and variations in appearance of the surface of the glass under the accelerated weathering conditions of the climate chamber produced structures similar to those found on original glasses; evidence was found of corrosion products and gel layers, and the chemical composition of the weathering products was basically comparable. There were clear differences however in the corrosion crust adhesion mechanisms. The production of corrosion-product and gel layers through weathering in the climate chamber is dependent on the chemical composition of the model glass used. The extreme sensitivity to corrosion of glasses of type 1 was clearly shown, while hardly any corrosion damage was detected on model glasses of type 2. Differences in the states of the surfaces of model glasses arising from the technology of their production methods (such as surfaces produced by rolling, fire polishing or mechanical polishing) were no longer detectable after four weeks under selected conditions.
The fact that the corrosion products lie loosely on the surface permitted the assumption that their mineral composition differed from that of the weathering layers that built up on original glasses over long periods of time. Phase localization of the products obtained on model glasses confirmed this expectation. The corrosion products consisted predominantly of syngenite, with small quantities of fine crystalline gypsum. Qualitative assessment of the proportions of the corrosion products, by means of X-ray crystallography on samples of powdered corrosion layer from a model glass after four weeks of weathering in a climate chamber, showed the proportion of syngenite to be more than 90%. The corrosion layers only lay loosely on the surface and could be removed easily mechanically. For some model glasses, it was possible to achieve a greater degree of adhesion by treating them thermally for 3 hours at 300°C after a complete weathering cycle; with glasses of other composition the stability of the corrosion crusts was increased by moistening the surface with water periodically. Potassium is removed from the syngenite by means of these additional interventions, and the higher proportion of gypsum leads to a stronger encrusting of the surface.
Investigations into Browning
The causes of various types of browning were outlined in section 2.3. Where browning results from manganese compounds, glass can be rendered more transparent by treatment with hydrazine (Fitz 1981; Perez y Yorba 1984). Because of the risks associated with this type of treatment, the need arose for further investigations, concerned on the one hand with the safety of adopting these methods of brightening, and on the other hand with addressing unanswered questions about the reasons for and mechanisms of browning. Within the context of this project, one of our aims was therefore to simulate browning on model glasses, and to carry out experiments to explain the reasons behind browning and its processes, as well as to examine the question of possible damage through hydrazine treatment.
For these experiments, model glasses were chosen whose composition was similar to that of original glasses that had browned. The gel layers and corrosion crusts obtained on them in the laboratory were similar to those on the original glasses (figs 6 and 7). Two model glasses were visibly browned across the whole surface after being weathered for 10 weeks. A white corrosion crust had built up, which consisted of c.10% by mass of gypsum and c.90% by mass of syngenite. Only after the crust had been removed was the browned glass visible.
From the results it was possible to draw conclusions concerning the influence of chemical composition on the model glasses that had experienced browning under the given conditions of weathering in the climate chamber. Clearly, the structure of the glass played a role in the browning mechanism. Glass is built up of tetrehedral units, so-called network builders (SiO2 and Al2O3 in the relevant glasses here), and network-modifiers, which are normally arranged in octahedral units (e.g., K2O, NaO, CaO and MgO). The network-modifier and network-builder contents of glass determine its characteristics, and thereby its tendency to brown. Characteristic of the compositions of these glasses is a low earth-metal content and a high alkali content; 0.6% by mass is already sufficient for browning to occur. All browned model glasses contained between 1–4% by mass of P2O5. The iron-oxide content appeared to play a subordinate role. Ratios of earth metals to alkalis and of network-builders to network-modifiers could be given that were specific to the glass. In the browned model glasses the ratio of earth metals to alkalis lay in the region of 10:6 – 10:8, and that for network-builders to network modifiers around 13:10, where manganese oxide and phosphorus oxide were both present simultaneously.
The corrosion products that resulted during browning could not be identified in every case. The spectra of manganese dioxide (MnO2) and the corrosion products on the glasses were not comparable. The manganese compounds present that cause browning must therefore not be oxides but rather salts. The manganese phosphates, whose presence has been suspected for a long time (Perez y Yorba 1984) could not be demonstrated in infrared spectra, because the vibrational bands of the other crystalline compounds (gypsum, syngenite) conceal any phosphate bands that might be present.
During the course of earlier investigations (Müller, Pouillon, Bochynek and Mehner 1986; Müller, Bochynek, Mittelstädt and Pouillon 1990) one way of treating several medieval glasses, some of them extremely browned, was revealed to be a method of brightening the glass considerably without visible damage to the painting. In qualification, it should be noted that although treatment with hydrazine hydrate leads to a chemical reduction in the manganese compounds (Mn4+ to Mn2+) in the gel layers, thereby reducing their colouring effect, it does not remove them. It was possible to demonstrate that treatment with hydrazine hydrate leaves the total manganese content practically unchanged. A browned piece of glass was placed in a 25% solution of hydrazine hydrate for 6 hours, and then cleaned off with distilled water and dried. Presence of the elements K, Ca, Si and Mn was determined by analysis of a section perpendicular to the surface. The distribution of the elements in the section through the surface is shown in fig. 9 (electron microprobe linescan). It shows that the high manganese contents of the gel layer remained unchanged. One must therefore expect renewed browning in the future as a result of reoxidization taking place over long periods of time.
One possible way of avoiding this consists of leaching the manganese compounds out by means of a 1% solution of formic acid. The damage potential of this method was tested through an appropriate treatment of model glasses, which showed that the method definitely had to be ruled out. While treatments of model glasses with hydrazine, even for an exposure time of up to 24 hours, followed by washing with distilled water did not produce a significant change, and in the event not a serious one, when compared with untreated model glasses, it had to be accepted that treatment with a 1% solution of formic acid (pH = 4.4) resulted in clear damage to the glass after only 5 minutes, and in excessive damage after 20 minutes.
If no interventions can be found for the removal of manganese, then the actual extent of the danger of reoxidization should be determined, at least approximately, through the simulation in the climate chamber of exposure over a long period of time. Appropriate experiments were carried out on several model glasses. In every case browning reoccurred within 4 weeks on the model glasses that had been treated with hydrazine to the level encountered before they were brightened. Coating the brightened glass samples with Paraloid B72 or with one of the Ormocers produced by the Fraunhofer Institute guarantees that even after 10 weeks of weathering in the climate chamber there is still no visible instance of browning. The conservation effect, particularly of Ormocer coatings (Fuchs, Römich, Tur and Leissner 1991), is clear. Sealing the surface of glass that has been treated with hydrazine with a coating opens up a second possible way of avoiding the reoxidization process.
Investigation into Coating with Sol-Gel
The gel layers that build up on medieval glasses through the leaching of alkalis and earth metals vary considerably in their extent and structure (Müller, Torge and Adam 1995). What is common to all of them however is the fact that they all exhibit defects, detectable mainly as cracks of varying width, as a result of the differences in their chemical composition compared with the gel layer and the different concomitant spatial requirements. Fig. 10 shows clearly three types of crack: relatively broad cracks running parallel to the surface, which eventually lead to the flaking off of parts of the gel layer in clumps; cracks running perpendicular to the surface, which allow corrosive substances to penetrate deep into the glass through the gel layer; and finally cracks spreading out into the core glass, along the edges of which the leaching process can be detected. Although the crack-free portions of the gel layer clearly act as a barrier to further hydrolytic attack on the glass, a new surface is created by the crack and thereby contact with the surrounding environment.
Experiments were conducted on model glass whereby surface structures were produced similar to those on medieval glass through weathering in a climate chamber, and a coating subsequently applied by means of sol-gel technology, with SiO2 being applied by means of an aqueous solution of tetraoxysilane (Dislich, Hinz, Arfsten and Husmann 1989). At the basis of these conservation experiments lay the idea of covering the cracks, or even better, filling them completely or at least partially, in order to prevent access to the core glass, thereby slowing the progress of corrosion; at the same time, it was hoped that the tendency of the gel layers to split off would be reduced.
The sol was applied by a dip-coating process, whereby the thickness of the layer could be regulated by the speed at which the glass was withdrawn from the sol. The thickness of the resulting SiO2 gel layer after drying amounted to 1µm at the most. The extent to which the sol had penetrated and thereby filled up the cracks in the gel layer could not be determined. The conservation effect of the coating was clearly demonstrated by infrared measurements on weathered samples, some of which had been subjected to the sol-gel treatment and others that had not.
The question of whether the concept of conservation through sol-gel treatment can be realized in practice, and the extent to which it justifies the expense involved, has not been answered definitively. In theory, the protective effect afforded to model glasses by sol-gel coating can be demonstrated, but the translation of these results onto originals may well be extremely difficult. Before any coating is applied, all corrosion products must be removed completely. An effective treatment would be to develop a coating technology that allowed the external face of a complete panel to be treated with having to remove the individual pieces from the whole panel. Assessment of the protective effect on originals can only be researched through pilot studies on originals in situ. Sol-gel coating however is not an alternative to well-functioning external protective glazing, though it could be taken into consideration sometimes as an additional measure. It should be mentioned that the microscopically thin layer remains bonded with the glass and can never be removed. This series of examples of research could be continued, but is brought to an end here for reasons of space. Priority should be given to the presentation of the results of investigative work that can be assessed by a restorer.
4. Micro-organisms on Historical Glass
The damage caused to historical glass by weathering can be seen to result from the influence of physical, chemical, and (as will be seen in the following) biological factors.
In scientific investigations to date, attention has been focused mainly on the external face of the glass (Marschner 1985; Newton and Davison 1989). Indeed, corrosion resulting from the damage caused by increasing levels of pollution in the atmosphere is at its most striking here. Thick encrustations arising from the build up of Wetterstein and browning cause windows to become dark. In addition to this, one must take into consideration the development of pitting and cracks that lower the mechanical stability of the panels, thereby endangering valuable material heritage.
Although there have always been indications, even if only occasionally, that bacteria and fungi further the decay of glass, only relatively few studies have concerned themselves with the attack of microorganisms on glass. This fact is hardly surprizing, since microbiology is often an area unfamiliar to material specialists and restorers, and the necessary proof methods are not in the standard repertoire of restoration-workshop procedures.
In recent times, research into damage caused by microbial action has become more urgent, in relation to the restoration of the Cistercian churches at Haina and Altenberg. As a result, in the context of this research project, particular attention was paid to the problems of attack by mould. Three questions were at the forefront of thinking in this respect. (1) How does a fungal attack on glass occur? (2) What types of corrosion damage do fungi cause? (3) Which restoration and conservation measures could be of assistance in this area?
In order to answer these three questions, three mutually complementary part projects were undertaken. (1) The accelerated corrosion of model glasses by means of selected species of fungus was investigated. The fungi selected were those whose presence on historical glass had been demonstrated by the Institut für Geomikrobiologie at the University of Oldenburg for monuments such as Tours Cathedral and the Church of St Catherine in Oppenheim. The areas of investigation were damage phenomena, mould growth, and metabolic products. (2) In order to understand damage mechanisms more precisely, simulation experiments were carried out with different organic acids. These organic acids are one of the components of the metabolic products of fungi. (3) Detailed studies of microbial attack were undertaken on various historical glass objects. Following on from this, research was undertaken into corrosion damage caused by microorganisms.
4.1 Micro-organisms
Micro-organisms on Historical Glasses
Micro-organisms include bacteria, fungi, yeasts, algae and lichens. The results yielded by research into the windows of Tours Cathedral in France (Krumbein, Gorbushina and Rudolph 1993) show that the percentual proportion of active live moulds is 3–9 times higher than the counts for living bacteria and algae. This finding was repeatedly confirmed by our investigations into historical glasses. As a result, our laboratory experiments concentrated on this type of microorganism.
In order to achieve the growth of fungi, a whole series of preliminary requirements have to be fulfilled; these so-called abiotic factors are set out in fig. 11. The most important factors that should be pointed out are the organic and mineral nutrients, and high levels of moisture in the environment; in addition, the external temperature, the pH-value of the substrate (acid/neutral/alkali), light, and the composition of the surrounding atmosphere play a role (Adan 1994). Various elements have to present in the nutrients, without which the growth of fungi is not possible: macroelements (C, H, N, S, P), macrominerals (Na, K, Mg, Ca), and a whole series of metallic trace elements (Mn, Fe, Cu, Zn).
In addition to the humidity of the air, the water-absorbing potential of the surface has to be taken into consideration. This depends on porosity, the incorporation of water into the structure of the glass, surface absorption (chemical and physical), and the biofilm already present on the glass, which has high water-retaining capabilities. A measure of the water content of the surface is the water activity aw, which allows us to express the amount of water that is effectively accessible by micro-organisms and which plays a decisive role in microbial colonization.
Glass itself cannot be used as an organic nutrient, but it can certainly act as a source of minerals and trace elements. In the case of historical window glass, the supply of organic nutrients usually results from organic materials present in dirt build-up, natural polymers derived from paint layers, or the remains of putties. Conditions that facilitate swift biological growth are encountered principally on the internal face of glass. Sufficient levels of water, the fundamental prerequisite for microbial growth, are usually provided by the cyclical formation of condensation. Furthermore, the build-up of dust and organic remains in internal spaces is generally higher than in external areas, allowing the mould and bacterial counts to increase, and preventing a noticeable reduction either by air movements or the destructive effect of ultraviolet rays in sunshine. If the conditions outlined above are present, then above-average growth of fungi on glass surfaces can result. Windows in unheated, damp churches without protective glazing are in particular danger. Although unheated interiors can be relatively cool (c.15°C in summer), extreme levels of fungal growth can occur on account of correspondingly higher levels of humidity and the availability of organic nutrients, as was shown for the north window of the former convent church at Haina (Drewello 1998).
Identifying Moulds
In order to detect moulds it is necessary to know some basic facts about the structure of fungi, their modes of reproduction, and their metabolic products. Everyone knows ‘moulds’ as growths on rotten foodstuffs. Most often, they consist of cotton-wool-like networks of branching fungal threads. These networks, known technically as mycelia, cannot always be detected by the naked eye. If nutrient levels are low, as is usually the case with glass, a microscope has to be used as an aid. Only at magnifications of more than x100 can the vegetative structures of most fungi be identified as such, and distinguished clearly from other apparently fibrous forms of dirt.
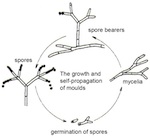
Fungi reproduce asexually. The commonest form and method of reproduction is by spores. These single-celled objects develop at the ends of spore-bearers, specialized structures that emerge from the mycelium (fig. 12). Since the spore-bearing structures vary greatly in appearance, and because spores have characteristic colours, these traits constitute a basis for distinguishing fungi from one another and classifying them. Spores exhibit a range of coloration in white, green, red, brown or black. When they have achieved maturity, they are ejected and can be easily transported by air currents. If they fall on suitable substrate, they germinate and grow new mycelia; these in turn can produce new spore-bearing structures. During growth, a range of moulds are able to produce organic acids, including citric acid, formic acid, malic acid and oxalic acid. The acids are excreted along the filaments of the mould, also known as hyphae. These chelating acids are capable of attacking the glass surface and thereby corrode it. In this way, the mould network can leave etched traces on the glass. Mould damage to the glass surface can therefore be demonstrated, even when the mould is no longer present. Examples of this are the damage caused to optical lenses in microscopes and other optical instruments in tropical climates.
The Current State of Knowledge
It has been known since as far back as the 1960s that particular patterns of damage can occur through the effect of moulds. Building materials and glass are not only exposed to physical and chemical weathering, but under certain conditions microorganisms can be responsible for their destruction to a substantial degree. With Hyphomycetes, the main form of fungus responsible for corrosive attack on glass, we are not dealing with ‘exotic’ fungi of any sort, but with different manifestations of species of the genera Aspergillus, Penicillium and Cladosporium, which are ubiquitous and in favourable circumstances lead to mould growth.
The damage resulting from mould attack is caused by the fungal network, which often reaches far across the glass. During growth, the filaments surround themselves with a continuous glycocalyx, the width of which can vary, which contains the organic acids and chelating agents. As a result of chemical attack by these substances, the surface of the glass becomes etched along the whole length of the mycelium (by means of the mechanisms already described), and so-called Pilzkorrosionsfiguren (shapes resulting from fungal corrosion) are produced.
The speed at which this type of damage can occur, under favourable environmental conditions such as high humidity and optimal temperature, can be enormous. Theden and Kerner-Gang (1964) demonstrated that an etched pattern formed by the network of hyphae can occur after an incubation period of only 24 hours. These Pilzkorrosionsfiguren, which produce a change in the refraction of the glass surface where it has been damaged, mean that contaminated lenses in binoculars or microscopes become dull and opaque, and therefore unusable. At the same time, the type and spread of these etched figures and patterns depends just as much on the type of glass as on species of fungus attacking it. These corrosion patterns are also observed on medieval panels (fig. 13). Transparency is greatly reduced by them, and the whole optical effect is rendered false and strange. The process can be so widespread that the result is a browning resembling that described in section 2.3 here.
One example is the convent church at Hauterive in Switzerland; here all the panels of medieval glass are overgrown with a black mould (Taeniolina deightonii) and have suffered browning (Kaiser, Trümpler and Raschle 1996). During active periods, other sorts of fungi excrete crystalline products that are deposited on the corrosion layer. In such cases we are talking generally about the salts of organic acids that are not easily disoluble formed as a result of metabolic processes, e.g., calcium oxalate or ferrous gluconate, which reduce the transparency of the glass and detract from the artistic effect of the glass-painting.
Proof for damage caused by microorganisms in particular is the presence of unusually high levels of sulphur, manganese and phosphorus within the corrosion layers, as these elements are accumulated and excreted biogenically through metabolic processes. Like the pitting in many instances of corrosion that has inorganic causes, there is a concentrated formation of pits with bio-pitting (see fig. 14). The formation of ‘rinds’ in concentric rings points to microbial attack. In section, the internal structure of the holes has a characteristic make-up, since the growth cycles of microorganisms, manifested in layered deposits of not easily soluble compounds, mirror the concentric rings around the original point of biogenic attack (Pohlman and Oberlies 1962; Kerner-Gang and Schneider 1959; Theden and Kerner-Gang 1964; Kaiser, Trümpler and Raschle 1996; Drewello 1993; Weissmann and Drewello 1996; Gillies and Cox 1988; Krumbein, Urzi and Gehrmann 1991).
4.2. Simulation of Microbial Attack
Experiments with Model Glasses and Microorganisms
The compositions of medieval (window) glasses have a few peculiarities that are of import for the model glasses used here. Naturally, one cannot compare either the technological or chemical conditions of the middle ages with the current state of technology. The production of glass from sand and wood ash explains the high proportions of elements such as P, Fe, Mn and S, impurities that became part of the mix unintentionally and whose percentual proportions in the composition vary greatly depending on the location of the glassworks and the raw materials used (Besborodov 1975; Geilmann and Jenemann 1954).
One characteristic of medieval glasses is the high proportion of network-modifiers, among them potassium (which in modern glasses is almost completely replaced by sodium), and correspondingly the reduced proportion of network-builders such as SiO2, which builds the glass matrix and is a very important factor in the chemical stability of modern glasses. The high proportion of network-modifiers and the low network-builder content (c.50%) can be traced back to the technology of those times, which was not capable of producing the high temperatures required for melting found in today’s manufactories, and attempted to mitigate this problem by increasing the proportion of flux.
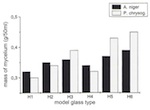
A series of model glasses was produced in which, starting from a basic composition, the potassium-oxide and calcium-oxide contents were varied for each by 2% by mass (Table 2). Starting with glass with potassium-oxide and calcium-oxide contents of 26.5% by mass and 16.5% by mass respectively (H1), for each new glass the proportion of K2O was reduced by 2% by mass and that of CaO increased by 2% by mass, until the relative proportions of these elements was the opposite of what it had been (16.5% by mass of potassium oxide and 26.5% by mass of calcium oxide, H6). The selection of microorganisms was limited to three typical moulds: Penicillium chrysogenum, Botrytis cinerea, and Aspergillus niger.
Penicillium chrysogenum manifests itself as flat, velvety yellowy-green populations, often with a yellow underside. This species is a very widespread soil fungus that can also be found in extreme locations (e.g., on glacial ice in Alaska) (Domsch and Gams 1930). Botrytis cinerea is found especially in upper soil layers, but not as a high proportion of the total population of fungi. The species is among the fast-growing fungi and can also exist in difficult conditions (e.g., pH-value = 2–8) (Domsch and Gams 1930). Aspergillus niger is a widespread spoiler of foodstuffs. Its specific name derives from the black colour of its fruiting bodies. It is employed industrially in the production of citric acid, up to 90% of which is produced by fermentation (Reiss 1986).
Experiments were carried out under sterile conditions on submerged cultures. Each of three glass samples (measuring 20 x 15mm) with cut and polished surfaces was coated with a mineral medium containing glucose and then contaminated with spores from each of the three fungi. Over the 36-day period of the experiment, the cultures were incubated under static conditions in an incubator (at 21°C). The pH-value of the reaction solution was determined through cyclic measurements, and the dry mass of the biogenically synthesized protoplasm (mycelial mass) was determined gravimetrically after 36 days and analyzed for crystalline components.
In order to determine the characteristics of the glass-specific conversion zones (gel layers), each of six sample bodies was immersed in 100ml of mineral medium, and then removed at regular intervals and examined by means of light microscopy, electron microscopy, Fourier transform infrared spectroscopy, electron-microprobe technology, and X-ray diffractometry.
| Oxide | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | KD1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.0 | 48.75 |
| K2O | 26.5 | 24.5 | 33.5 | 20.5 | 18.5 | 16.5 | - | - | 16.5 | 16.5 | 23.5 |
| CaO | 16.5 | 18.5 | 20.5 | 22.5 | 24.5 | 26.5 | 16.5 | 26.5 | 26.5 | 26.5 | 17.5 |
| Al2O3 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 1.75 |
| P2O5 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 2.0 |
| MnO | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 2.4 | 4.8 | 1.7 |
| Na2O | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 27.5 | 17.5 | 1.0 | 1.0 | - |
| - | - | - | - | - | - | - | - | - | - | - | 0.4 |
| - | - | - | - | - | - | - | - | - | - | - | 4.0 |
| - | - | - | - | - | - | - | - | - | - | - | 0.4 |
The Connection between Mycelial Growth and Glass Composition
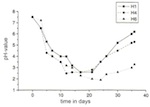
Over the period of the experiments, significant differences were established for the individual model glasses with regard to the amount of biogenically synthesized protoplasm. For all species of mould it can be said that the mycelial mass increases the higher the level of CaO and the lower the level of K2O. With model glass H4 Aspergillus niger and Penicillium chrysogenum deviated from the curve plotted on a graph (of relative minimum amounts). Development of the mycelial mass, which is dependent on the type of glass, corresponds with changes in pH-value, which are time-dependent. As a result, the pH-value of the reaction solutions, with their rising CaO content, was pushed into the acid range after 36 days: the solution for model glass H1 had a pH-value of 6.5, and that for H5 a pH-value of 3.0. The experiment again showed anomalies in relation to model glass H4 (figs 15 and 16).
Conversion Layers
For all the samples taken during the first 18 days there was a swift increase in the size of the alkali-depleted gel layer, when measured at the boundaries. For Aspergillus niger the maximum thickness of the layer was c.80µm with model glass H1. With the oxalic-acid-producing fungus the strength of the conversion layers decreased continuously, from K2O-rich glass to CaO-rich glass, and with model glass H6 amounted to 50% of the value measured for H1 (fig. 17). Model glass H4 deviated from this linear progression. As the experiment continued, the thickness of the gel layer increased unevenly as the minimum pH-value increased. Towards the end of the period of the experiment, the gel layer regained strength. With Aspergillus niger the conversion layer of model glass H1 possessed an internal structure characterized by cracks running both perpendicular to and parallel to the surface. Calcium oxalate was deposited in the perpendicular cracks, and at the ends of the cracks in the core glass semicircular leaching zones were detected. The number of cracks running parallel to the surface increased as the CaO content increased. With model glass H6, only cracks running perpendicular to the surface were found. A remarkable discovery was the sharp division into two of the gel layer, into a layer c.3µm thick in contact with the fluid phase that was extremely depleted of the elements Ca, K, Fe, Mn, P and Al, and a de-alkalized layer beneath spreading to a depth of c.30–70µm.
Discussion
From the progression of mycelial growth and pH-value, it follows that the synthesis of biomass and acid metabolites is influenced by the composition of the glass. There was a continuous increase in the mycelial mass as the CaO content of the model glasses increased and the K2O content decreased. This is probably the result of more favourable growth conditions or a lengthening of the stationary phase in the life cycles of the microorganisms. The deviations with model glass H4 were surprising, with regard to the mycelial mass, as well as the progressions in pH-value and strength of the conversion layer (with the percentages by mass for K2O and CaO being 22.5 and 20.5 respectively). The significantly lower values lead one to suspect that the prerequisites for growth for certain microorganisms were unfavourable. Only further experiments will determine whether ion exchange at the phase boundary or the transport of K and Ca are noticeably hindered with specific K/Ca relationships.
From the development of the conversion layers it can be deduced that there is an almost linear relationship between the durability of the model glasses compared with biochemical attack and K2O/CaO proportions. Despite better growth of the mycelium, CaO-rich glasses exhibit much shallower dealkalized layers. The development and inner structure of the conversion zones is highly dependent on the components of the glass, as well as the type and concentration of the biogenically produced organic acids. Deposits of calcium oxalate in the gel layers of the K2O-rich glasses and in the semicircular leaching zones found at the ends of cracks running perpendicular to the surface demonstrate that cracks must have developed in the fluid phase on account of tensions within the dealkalized zone. The clear division into two of the gel layers indicates that different reaction mechanisms exist for microbial deconstruction of the glass surface.
Simulation of Microbial Attack with Organic Acids
Using model glass H6 as an example, the acid spectrum of the reaction solutions that had been contaminated with moulds was determined after 12 days by means of gas chromatography (see Table 3). The results show that the acids principally responsible for damage to glass are oxalic acid, gluconic acid and citric acid. In order to study these complex processes over time, model glass H3 was taken as an example and separate samples were subjected to chemical attack by the three acids.
For the purposes of comparison, the following were also used: an inorganic acid (hydrochloric acid), a strong organic complex-builder (EDTA), and a non-complex-building organic acid (acetic acid). The experiments were carried out with three different concentrations (0.1, 0.01 and 0.001 molar solutions) for each acid. The process of corrosive attack was monitored using various procedures. The concentrations of leached ions in the solution were determined by means of flame spectroscopy (for K) and inductively coupled plasma atomic-emission spectroscopy (for Si and Ca). The thickness and morphology of the corrosion layer was investigated using a scanning-electron microscope. The initial status of the corrosion was established using Fourier transform infrared-reflection spectroscopy. Long-term experiments were likewise carried out over a period of 36 days. The loss of mass as a result of corrosion was determined by weighing the samples before and after treatment.
| Components | Penicillium | chrysogenum | Botrytis | cinerea | Aspergillus | niger |
|---|---|---|---|---|---|---|
| (mg/l) | mol% | (mg/l) | mol% | (mg/l) | mol% | |
| oxalic acid | 6.956 | 0.046 | 7.749 | 0.052 | 299.917 | 2.000 |
| glycerine | 19.696 | 0.128 | 285.53 | 1.860 | 463.233 | 3.021 |
| Bernstein acid | 1.054 | 0.005 | 3.819 | 0.019 | 0.852 | 0.004 |
| fumaric acid | 0.394 | 0.002 | 0.309 | 0.002 | 0.397 | 0.002 |
| lactic acid | 2.286 | 0.015 | 3.605 | 0.024 | 0.799 | 0.005 |
| glutaric acid | 0.857 | 0.004 | 0.247 | 0.001 | 0.572 | 0.003 |
| malic acid | 0.149 | 0.001 | 1.763 | 0.008 | 0.784 | 0.004 |
| 2-oxoglutaric acid | 0.663 | 0.003 | 2.037 | 0.008 | 10.977 | 0.041 |
| cis-aconitic acid | 0.656 | 0.002 | 1.622 | 0.006 | 27.440 | 0.095 |
| citric acid | 0.745 | 0.002 | 4.805 | 0.015 | 16.645 | 0.052 |
| galacturonic acid | 1.998 | 0.006 | 7.564 | 0.023 | 97.5 | 0.301 |
| gluconic acid | 653.262 | 2.000 | 1998.436 | 6.116 | 7647.949 | 23.407 |
Short-term Experiments
The corrosive effect of acids could be demonstrated after only a few seconds on the infrared spectrum. Both characteristic reflection peaks at 1050cm-1 and 925cm-1 changed clearly during the acid attack. In the case of oxalic acid at a concentration of 0.1mol, the 1050cm-1 peak moved by Δν = 50cm-1 to higher wave numbers within a second. The intensity of this peak tails off strongly after 1 second and within 1 minute, only to increase again after 10 minutes. By contrast, the 925cm-1 peak decreased continuously throughout treatment. After a leaching period of 100 minutes, no further change in the spectrum was observed. This means that after this time the thickness of the corrosion layer has reached the information depth of infrared-reflection spectroscopy. At a wavelength of 10µm this corresponds to a depth of c.2.5µm. The spectrum showed the traits characteristic of silicate glass. This discovery points to high levels of leaching of K and Ca ions. Citric acid and gluconic acid show similar corrosion behaviour, but with a lower rate of leaching. The following tables show the percentage decrease in the intensity of the 925cm-1 peak Δ1 as a percentage.
| 0.1mol | 0.1mol | 0.1mol | |
|---|---|---|---|
| acid | oxalic acid | citric acid | glyconic acid |
| Δ1% | 46 | 30 | 29 |
| 0.1mol | 0.1mol | 0.1mol | |
|---|---|---|---|
| acid | oxalic acid | citric acid | glyconic acid |
| Δ1% | 18 | 6 | 5 |
For short-term attack it was possible to derive the following gradation: oxalic acid >> citric acid > gluconic acid.
Long-term Corrosion
The length of the long-term corrosion experiment amounted to 36 days. During the chemical attack, the pH-value climbed continuously, and the nature of the attack changed from pure leaching to breaking down the network of the glass. With the 0.1mol acids the leaching mechanism prevailed for the entire duration of the experiment. With the 0.01mol acids network breakdown prevailed after 18 days, and with the 0.001mol acids after only 12 days. Fig. 18 brings together the results for loss of mass for the 0.1mol acids. In this case, oxalic acid had the strongest corrosive effect compared with the five other solutions. The situation for the 0.01mol acids is on the other hand completely different (fig. 19). At this level of concentration the strongest attack was by the EDTA, followed by citric acid. In comparison with the 0.1mol solution, the intensity of the chemical reaction of the 0.01mol oxalic acid was reduced by a factor of about 100, and showed the least corrosive effect.
The results of the long-term corrosion experiment for the metabolic products of moulds can be summarized as follows.
| 0.1mol: | oxalic acid >> citric acid > gluconic acid |
|---|---|
| 0.001mol: | citric acid > gluconic acid > oxalic acid |
In order to explain this contradictory behaviour, the corrosion layers were investigated in detail with a scanning0electron microscope. Figs 20 and 21 show the corrosion layers produced after being treated for 6 days with acetic acid and oxalic acid. The acetic acid produces an almost homogeneous hydrate (gel) layer. In this layer two different sorts of crack could be demonstrated: type A occurred from an early stage during leaching, and could be followed directly with a light microscope, while type B was the result of the drying out process for the gel layer after attack.
The 0.01mol oxalic acid produced a thicker hydratized layer over the same period of 6 days. In addition to the formation of this layer, deposits of calcium oxalates were observed on the surface. These crystalline precipitates also build up in the deep cracks, which leads directly to mechanical destruction of the glass and explains the high loss of mass with the 0.1mol oxalic acid.
Cracks: Origins and Patterns
As has been shown, the formation of cracks had a dominant influence on the destruction of the glass surfaces. Their formation was observed in simulated conditions in damage caused by humidity and corrosive gases as well as with microbial corrosion. Cracks formed both on the glass’s being subjected to corrosive attack (type A) as well as during the drying-out phase (type B). In the first case, two types of crack pattern could be distinguished: cracks that spread along the surface in straight lines (fig. 22b), and hen’s-foot-like cracks (fig. 22a). The first type of crack pattern is typical of leaching by inorganic acids (HCl) and acetic acid, and during drying out. In both cases, tensile stresses trigger the crack formation. Tensile stresses build up through the ion-exchange process between K+ and H3O+ ions, because K+ ions have a longer ionic radius (0.138nm) compared with H3O+ (0.114nm). The replacement of potassium ions with hydromium ions reduces the molar volumes, and the surface of the glass shrinks. This shrinking, like the drying-out process, creates tensions in the surface and triggers the formation of cracks when a critical level is passed. The cracks can spread out both laterally across the surface of the glass, as well as reaching deep into the glass, to the boundary between the gel layer and the core glass (fig. 20).
Crack patterns, as fig. 22a shows, are observed with leaching with oxalic acid and citric acid, which both exhibit a strong affinity towards Ca2+ ions. The origins of the triangular cracks are binodal phase separations in the glass. Localized Ca-rich phases could be demonstrated in the glass matrix with a transmission-electron microscope. The diameter of these inhomogeneities amounts to c.50nm. Ion exchange between one Ca ion and two hydromium ions leads to localized compression stresses, which build up tangential tensile stresses in the surrounding glass. For their part, these tangential tensile stresses can cause cracks that run radially to the local defect.
4.3 Case Studies on Microbially Induced Corrosion on Historical Church Windows
Biogenic Attack and Crust Formation: the Tree of Jesse Window from St. Patrokli in Soest (1160–66)
The Romanesque Tree of Jesse Window from the chapel of the Virgin in the northern arm of the transept of the church of St. Patrokli in Soest was probably executed in 1160–66. No restoration interventions are documented before 1863, though one suspects that there was at least one (not exactly datable however), when the vitreous paint was overpainted. In the years 1863–64, the glazing was partly restored and partly renewed by the Cologne glass-painter Friedrich Baudri. After being removed for safety during the war years 1942–43, there was a further restoration in 1948, executed by the Soest glass-painter Brauck, which included releading and extensive puttying. After the glass had been reinstalled, cold painting was applied to the internal face of the glass, which had a darkening effect. In 1974, it was decided to remove the glass again, because corrosion on both faces of the glass had reached a threatening level (Korn 1992). Since then, the Tree of Jesse Window has been in store at the Oidtmann Studio in Linnich. With the exception of short interruptions, the glazing in question was installed in the north transept of the church from the eleventh century until modern times. Measurements are not available for the internal climate, and no documentary evidence has come down regarding any protective measures undertaken, or of what type. Since being removed from the window, the glazing has been stored in a cabinet with a stable climate, at room temperature with a relative humidity of 40%.
Analyses of the compositions of the medieval and modern glasses are not available. From the dating of the glazing, the encrustations of gypsum and syngenite on the external face and the corrosion layers on the internal face (at least for the colourless and yellowish pieces), it can be inferred that the glass is a wood-ash glass typical of the period.
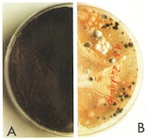
Despite its having been stored for twenty-five years in particularly low relative humidity, the internal face of the glass is remarkable for the high hyphal count of Hyphomycetes. After an agar impression had been taken, it was possible to determine the presence of 5–6 species of fungus belonging to the genera Cladosporium, Penicillium and Aspergillus, so-called moulds.
The medieval glass, particularly the colourless glass, is damaged to a considerable extent on both sides. On the external face Wetterstein crusts several millimetres deep have formed, and the internal face was covered with a tension-filled misshapen brown crust (fig. 23). When the 0.3–0.6mm-deep crust on the internal face was removed, a carpet of newly formed crystals came to light. The crust often did not form on the trace-lines.
The encrustation on the internal face, attack on the glass, and biogenic infestation cannot be considered separately here. The basic structure of the compact crust is a thick, branching network of hyphae. Embedded in this biogenically formed structure are inhomogeneous, organically bound agglomerates and pigments (iron oxides). Spectroscopic analysis of the organic portion showed that we were dealing with the remains of a coating that contained oil and probably protein. Among the other components of the crust were particles of glass; phases containing Pb, Cu, Mn, Si and Mg (pigments, dirt deposits); calcium carbonate; and considerable quantities of sulphates (gypsum, syngenite).
The number of crystalline phases within the crust also increased with increasing proximity to the glass surface. Similarly, the degree to which the hyphae were coated with sulphates also increased. The white underside of the matted layer consisted almost entirely of sulphates, while the brown topside was distinguished by a high proportion of brown/black organisms (‘black moulds’) and a considerable number of particles from the gel layer. The presence and distribution of sulphates underlines the capacity of the bio-crust to store air-borne contaminants and to intensify hydrolitic attack at a local level. The crust served as a crystallization space for compounds that were relatively easily soluble, and through the pressure of crystallization promoted the bio-physical breakdown of the corrosion layer.
Biogenic Attack and Browning: the Angel Panel and Sexfoil from the Church of Sts Cosmas and Damian in Goslar (c.1235)
Significant remains are preserved from the glazing of the late Romanesque choir in the Protestant market church of Sts Cosmas and Damian in Goslar. Among them are an ogival panel with a praying angel (fig. 24, the ‘angel panel’), and a hexagonal area of glazing with six doves arranged in a ring (the ‘sexfoil’). Both the angel panel and the sexfoil were housed in the Goslar market church until 1968. They both contain extensive stopgaps, and attained their current form only during earlier restorations, at least two of which were executed during the last 100 years (Müller-Weinitschke in Brinkmann et al. 1995). After the glazing was removed on conservation grounds in the 1970s, the panels were stored, without further restoration, in cases. In 1997, the angel panel was reinstalled in the church, in a side chapel.
The nature of the climatic conditions and damage to the glass until it was removed in the 1970s is not known. After the glass had been removed, it was stored wrapped in newspaper in a particle-board case. The storage conditions were not able to suppress the growth of fungi – as could be observed after unwrapping on the sexfoil, on which spot-like colonies of penicillin were to be seen.
Fig. 25 shows the extent of the medieval glass in the sexfoil (the areas marked red). Following analysis of glass samples, it was established that the original portions consisted of wood-ash glass typical of the period. The proportion of SiO2 amounted to 50%, and there were equal proportions of K2O and CaO. The presence of further constituents was also demonstrated: Mg, Al, P, Fe and Mn. The colourless glass contained higher levels of manganese, and the flashed glasses a certain amount of lead and copper. The inserted pieces fell into the category of soda-lime glass, typical of the late nineteenth and early twentieth centuries.
The angel panel and sexfoil exhibited above-average levels of microbial attack, with the proportion of fungi clearly exceeding that for bacteria (see figs 26 and 28: the blue markings indicate areas of fungal attack). Both the angel panel and the sexfoil exhibited much higher fungal hyphal counts on their internal faces than on the external faces. The extant medieval glass was colonised more extensively than the glass of the late nineteenth or early twentieth century. 13 sorts of fungus were isolated, the proportions of which varied greatly across the glazing. Species from 3 genera of fungus were found repeatedly: Rhizopus, Trichoderma and Penicillium. Some of the fungi that had been identified and assigned taxonomically were able to excrete organic acids, and had unusually high nutrient requirements. Their growth indicates the presence of a supply of organic nutrients. 2 of the 13 isolated fungi could be characterized as potential producers of melanin pigments. Microbial oxidation of manganese and iron cations was not observed, nor was the formation of metal salts.
The patterns of damage and rates of corrosion for the medieval wood-ash glass and the modern soda-lime glass vary in line with the chemical stability of their substrates. For the first glass very heavy browning was observed (fig. 27). The causes of the discoloration can probably be traced back to manganese deposits in the gel layers, as well as a firmly adhering biogenic coating (organic browning). The soda-lime glass exhibited no instances of corrosion or discoloration, although the glass had been exposed to weathering over a long period of time; this was indicated by the formation of sulphates (gypsum and anglesite on the lead matrix, anglesite on the glass itself) and sulphuric-acid attack on the lead and the putty. This chemically stable glass remained unharmed; breaks and deterioration in transparency were the only impairments.
Typical of the extant medieval glass were encrustations and a loss of substance. The glass was covered by a brown layer on the internal face, the craquelure of which pointed to the application of a restoration coating (see fig. 30, layer C in the scanning-electron microscope section). The coating was found on all the original glass, but traces were also found on the later additions, so it could not be more than 100 years old. After analysis of the organic components, it was established that the binding agent was probably a natural polymer with sulphur-rich proteins.
Examination with an electron microscope showed that the inhomogeneous layer was attacked and overgrown with fungi and had a depth of up to 200µm. Analysis of the solid particles and binding agent pointed to a biogenically decomposed coating, pigmented with iron oxides and with an oil and protein base, in which gypsum and calcite now predominate. The growth of penicillins on the internal and external faces of the glass was not even stopped during storage at the Erlanger Institut für Werkstoffwissenschaften (fig. 29). Halos of dampness developed around the growth centres, and beneath these and along the hyphae of the fungus the material of the glass was destroyed. The mycelium above the substrate contained calcium carbonate and calcium oxalate. That is was not exclusively the fungi, but also bacteria that had a role in building up the biomat was shown by the unusually high levels of sulphur and particularly phosphorus on the undersides of particles loosened from the gel layer, as well as on the coating applied to the glass after it was executed (see fig. 30, layer B in the section, image taken with scanning-electron microscope). The concentrations of S and P were accompanied by an above-averagely high bacterial germ count and a high iron content.
The changes observed in colourless medieval glasses can be attributed to several factors.
- A decisive factor in the colour effect of a window is what is known as manganese browning. This is caused by the deposit of brown manganese oxides and hydroxides in cracks caused by tensile stresses in the gel layer; the Mn2+ ions come from the network of the discoloured glass (see fig. 30, layer A). The mobilization of metals is promoted by the biocrust, in which the accumulation of damp and contaminants triggers a localised reaction.
- A second reason for the darkened colour effect is the extensive, uniformly thick 20µm layer B, whose porous structure and component elements (lead, iron, manganese and aluminium oxides, and silicates) provide evidence for a coating fired on at a low temperature. We are probably dealing with a restoration coating applied in the late nineteenth or early twentieth century. Layer B has the effect of restricting diffusion of light; compounds of phosphorus, manganese and lead were later able to accumulate on the topside and underside of this. On the topside there were shown to be high concentrations of sulphur that correspond only marginally with calcium.
- The third cause of browning was the biogenically attacked coating itself (C), which appears a very dark brown on account of the ageing of the organic binding agent, the extremely high levels of dirt on it, and the colour of the fungi themselves.
- Organic substances that were dark-brown to black in colour isolated in small quantities from the biocrust contributed to darkening of the colour. Investigation by infrared and electron-spin-resonance (ESR) spectroscopy showed these to be complex compounds with hydroxycarboxylic acids and aromatic alcohols. The broad ESR signal without any fine substructure indicates the presence of free radicals from aromatic systems, a possible indicator of (bio)polymers. These may be pigments (melanins) typical of the fungus, or cell components networked by oxidation following autolysis of the microbes.
The following may be indicators of the biogenic role played in corrosive attack on the internal face of the glass (which was protected from weathering) and in browning: the considerable thickness of the gel layer, the intense growth of acid-forming deuteromycetes, unusual concentrations of biogenic elements (potassium, sulphur, phosphorus) at the phase boundary, and the presence of biopolymers.
Biogenic Attack and Pollution: Panel 2b from the West Window of the Former Conventual Church of the Cistercian Abbey at Altenberg
The west window of Altenberg Cathedral was executed c.1400. The last major restoration was carried out in the 1970s by the Glasmalerei Oidtmann studio in Linnich. From the documentation drawn up at the time can be seen, among other things, the proportion of cracked original pieces, which on conservation grounds were coated on the external face with epoxy resin and then plated. As a result of the change in the thickness of the glass, it was necessary to relead the panel and reputty it. From 1994, the panels of the west window were temporarily removed from Altenberg Cathedral, while protective glazing was installed.
We have no precise information on the climatic situation at the west window. Immission measurements made as part of a research project in the 1980s showed high levels of moisture and corrosive substances on the inside and outside of the church (Fitz 1995). From 1994, the panels were kept in store at the Glasmalerei Peters studio in Paderborn, where the relative humidity was 60% and the temperature ranged from 18°C to 20°C.
The surfaces of the glass facing the inside of the church were found to be greatly contaminated (fig. 31). Irrespective of the composition of the glass substrate, refurbishment over time, and plating, downy colonies of fungus settled on the glass in a brown-coloured layer, which under the microscope was identified as a fungal network. The medieval glass had much higher hyphal counts than the replacement glass of the nineteenth century.
In contrast to the fungal attack by black moulds on the internal face of the glass (fig. 32), the external face was home to a balanced population of microflora in which no group of organisms was dominant, giving a heterogeneous mat of fungi, bacteria and yeasts.
On the internal face 3–4 species from the genera Cladosporium, Ulocladium, Alternaria and Aspergillus (black moulds) dominated. The dark colour of the fungal mycelia and their associated fruiting bodies determined the brown coloration of the coating, which is exacerbated by deposits of dirt. The macroscopically visible individual colonies could be assigned to various species of the genera Mucor, Penicillium and Chrysonilia. A good half of the isolates were able to excrete organic acids. In the plated sections (between the plating glass and the epoxy resin, and between the resin and the original glass), as well as on the synthetic materials, no fungi were detected, though bacteria were found.
In comparison with the external faces of the panels, which were cleaned in the 1970s and are still clean today, the internal faces gave the impression of having built up considerable amounts of dirt. The thickness of the ‘dirt layer’ depended on the type of glass: the medieval portions were covered with a thicker layer than the modern replacement glass. No differences were detected across individual pieces of glass: neither the trace-lines, nor the leading, nor the position of pieces within the window had an effect on the thickness of the layer. Differences could only be attributed to the variation in surface texture between the original and the modern glass, because medieval glasses are noticeably rougher than the nineteenth-century ones on account of the weathering and ageing processes. When the panels were re-puttied, a considerably larger quantity of putty remains stayed on the corroded original glass. The increased supply in the carbon source promoted growth of the fungal population, which in turn increased corrosion damage to the chemically unstable wood-ash glass. Dirt layers and colonization by fungi reduced the transparency of the paintwork considerably, as was evidenced by the cleaning of a sample (fig. 33).
Once the brown surface layer had been removed, the white putty remains became visible, the organic part of which was linseed oil. Only traces of oil were still to be found beneath the brown layer, but numerous complex organic compounds were detected. Quartz, calcium sulphate, calcite and weddelite (calcium oxalate dihydrate) were found in crystalline state in the biocrust and the putty. The calcium oxalate is clearly of biogenic origin, because it does not occur in purely mineral environments, whereas the presence of calcite can go back to the fungi, the putty, or abiotic factors, such as the precipitation of atmospheric carbon dioxide. The following manifestations and mechanisms of biocorrosion could be demonstrated from the Altenberg panels.
- (a) The reaction of oxalic acid with calcite from the putty. The putty captures the oxalic acid, whereby the calcium carbonate is converted by the formation of calcium oxalate and carbonic acid. Surplus carbonic acid reacted further with the glass matrix through ion exchange.
- (b) Direct attack on the wood-ash glass by biogenically produced oxalic and carbonic acid, the leaching of K+ and Ca2+ ions, and the excretion of calcium in either oxalate or carbonate form. This proposition is supported by the build-up of trace elements in the glass.
- (c) The excretion of easily soluble alkaline carbonates onto contaminated surfaces, which can lead to the displacement of the pH-value into the alkaline range by c.0.5, leading in turn to reactions that break down the network.
5. Recommendations Concerning Restoration and Conservation Measures
As was already highlighted in the introduction, the realization that historical stained glass can be populated by microorganisms and considerably damaged thereby is relatively new. As a result, tests and experiments as to which restoration measures can be adopted against mould attack are still in their infancy. It is therefore difficult at this current moment in time to make recommendations. As a result, the interventions listed below must be discussed and executed in close collaboration with restorers, and the results checked in long-term studies.
In order to obviate the considerable potential danger from microbes that exists for original glass, it is urgently advised that the glass surfaces be subjected to a careful but thorough cleaning of organic remains, and that the microbial infestation be removed. To remove the fungal network, any of the mechanical treatments in use today can be employed, after subjecting the network to thread-reducing pretreatment. After mechanical dry cleaning, treatment with ethanol to remove the remaining organic matter is promising. In order to minimize the risks to health through overexposure to fungus spores, it is recommended that extremely contaminated surfaces are treated with compresses that reduce and kill off the hyphae.
The use of chemical methods is still a wide-open and much discussed area. Research on the glass of Erfurt Cathedral focusing on microbial attack point to the fungicidal effect of organic metal compounds. Because of the copper frameworks there, copper compounds form, which have the effect of restricting the growth of microorganisms; this discovery probably explains the low level of microbial attack on the glass. In order to achieve long-term protection of the glass from fungal attack, there is discussion as to whether to include fungicide in consolidation agents (such as Ormocers) and to coat the glass with them. Coatings containing bee’s wax appear to have had a similar effect. Fumigation with ethylene oxide, as is often recommended, should be avoided on grounds of its toxicity. Treatment is costly and does not offer long-term protection. Physical treatments could be an interesting alternative, but until now have been too little researched. Among these are exposure to ultraviolet light, which kills off the hyphae, or treatment in an autoclave at 120°C and 1.2 bars of atmospheric pressure. A completely new treatment is the cleaning of glass with laser beams. This possibility is currently being researched within the framework of a project supported by the Deutsche Bundesstiftung Umwelt (the German State Environmental Foundation). In this project, ‘Laser Technology in Restoration’, the Fraunhofer Institute für Silicatforschung, the laser laboratory of the technical college in Münster, and the Institut für Glas und Keramik in Erlangen are working together closely with restorers from the Cologne and Erfurt cathedral studios in order to develop a treatment that is as practice-oriented as possible. One of the main concerns of the project is cleaning panels of microorganisms.
How to treat organically and inorganically produced browning poses a problem. One procedure, based on the chemical reduction of manganese compounds, attempted to provide a way of solving the problem of inorganically produced browning (Müller 1985). There are however still no significant long-term studies concerning the stability of the reduced compounds in a normal oxygen-rich atmosphere. Great hopes are being placed in laser cleaning for the removal of organically caused browning.
The most important and most pressing problem in relation to microbial attack on glass is however that of long-term protection of panels. In order to approach this problem, it is necessary to bear in mind the requirements for the growth of microorganisms, i.e., the abiotic factors, which are set out together in section 4.1 and fig. 11.
The most important intervention that can be identified is definitely the reduction of the aw-value, which correlates with the relative humidity of the surrounding environment. A primary effective intervention is the installation of protective glazing, as this prevents the formation of condensation (aw = 1) on the panels. Beyond this, attention might be focused on providing a regulated interior climate with a relative humidity of <60%. Of course, deposits of organic matter, which act as a source of nutrients for microorganism, are very hard to avoid.
This also means however that despite the preventative measures outlined above, panels should be checked by means of samples at regular intervals, e.g., by determining the germination rate. If these checks show up renewed microbial attack, one should consider recleaning or further measures.
6. Summary
All works and measures applied to historical glass within the context of consolidation, conservation and restoration executed in glass studios demand not only broad expert knowledge, an ability to empathize artistically, and technical skill. The restorer must also be keenly aware that cultural heritage requirements – retaining as much of the original substance as possible, providing the highest levels of protection possible from future attack, coming as near as possible to the original appearance, taking reversibility and precise documentation into consideration – cannot be fulfilled without the help of scientists.
Medieval glass suffers from substantial damage, at least on the surface, as a result of chemical, physical and biological factors. Alterations in the appearance of the glass require the implementation of cleaning and consolidation measures; interventions undertaken in earlier restorations often have negative consequences that have to be corrected; and finally current conservation interventions produce questions of a scientific/technical nature.
If problems are recognised by the restorer, and questions are put to the scientist as precisely and concretely as possible, the application of one or several research methods can be expected to supply answers that make satisfactory practical treatments possible.
Within the context of the project described here, attempts were made primarily to discover new possibilities for cleaning, treatment for browning, and conservation. For this purpose, existing methods were adapted for special requirements, and new treatments were developed. Their application was tested both on original glass as well as on model glasses produced in imitation of the original, with the result that today research and characterization methodologies are available that can be applied in line with the desired goal or purpose. These methodologies can be used as follows.
- to determine the chemical and mineral composition of glasses, surface layers and paint layers (element and phase analysis)
- to obtain images of surfaces by microscopy and electron microscopy (morphological characterization)
- to identify microbial attack on the surfaces of materials
- to research the sensitivity of materials to environmental influences, by simulating chemical, physical and microbial attack
With the help of these research methods, it was possible to do the following.
- characterize original stained glass from a material/technical point of view, and draw conclusions for its treatment
- assess cleaning methods for their possible impact on the original substance of the glass
- test new methods of chemical treatment of medieval glass with very solid crusts
- evaluate chemical methods for the treatment of browning
- make comparisons between the different conditions to which glass was exposed, for example by examining internal and external faces, or the local environmental conditions
Furthermore, preliminary approaches to solving numerous questions that had remained open until now have been found, and these should be developed in future research projects (including, among others, laser treatment, coating with sol-gel, the application of fungicides, fumigation, and contour fixing). The results of this project will however make a substantial contribution to the preservation of stained glass that is not yet sufficiently well protected.
In light of the condition of medieval glass (which is already often very bad, with advanced progress visible in the transformation of the material), and considering the potential for damage in the future from the local atmosphere (which is sometimes very rich in corrosive gases), it will be absolutely necessary for those working in cultural heritage, restorers, technicians and scientists to collaborate in future.
7. Acknowledgements
Authors: Wolfgang Müller, Manfred Torge, Karin Adam, Hannelore Römich, Rudolf Weissmann, Rainer Drewello. This text originally appeared as chapter 4 ‘Naturwissenschaft im Dienst der Restaurierung’ (pp. 65–99), in A. Wolff (ed.), Restaurierung und Konservierung historischer Glasmalereien, Mainz, 2000.
Publisher: Verlag Philipp von Zabern.
Translators: Joseph Spooner with Léonie Seliger.
Images: Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin (figs 1–10); R. Drewello (figs 11, 13–14, 22, 30–32); R. Weissmann (figs 11–12, 22); T. Heß (figs 15–17); W. Weibelzahl (figs 18–21); C. Müller-Weinitschke (figs 23–29); U. Drewello (figs 28–28, 33).