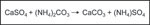Corpus Vitrearum Medii Aevi
Medieval Stained Glass in Great Britain
Your trail:
- Conservation
- Cleaning: a Balancing Act
Cleaning: a Balancing Act
(Hannelore Römich, Elisabeth Jägers, Manfred Torge, Wolfgang Müller and Karin Adam)
1. The Current State of Cleaning Technology
What are commonly thought to be layers of dirt deposited on historical stained glass can in fact have several causes: they can either derive from external sources (e.g., soot, dust, mortar and micro-organisms), or they may be caused by ageing processes in the glass itself. Early glass has often developed a 'gel layer' (an area over the core glass to which silicate molecules have migrated from the core of the glass, rendering that area harder and more chemically stable than the core), as well as opaque encrustations that substantially reduce the transparency of the glass (Fuchs, Patzelt and Schmidt 1989; Marschner 1985a; Müller 1992). For several centuries, stained glass was cleaned using mechanical methods (either tools or scouring sand), and also with acidic or alkaline solutions. Our ability to establish the extent to which such cleaning episodes have affected the original glass is somewhat limited, since the changes effected in the surface of the glass by invasive chemical treatments are often covered over by corrosion. Occasionally, however, such chemical cleaning processes do leave clear evidence (fig. 1).
Today's understanding of the cleaning process embraces two complementary aims - firstly improving the readability of the glass, and secondly slowing the ageing process of the historical glass itself. The act of removing deposits and encrustations resulting from corrosion should not endanger the artwork itself. The conservation mantra 'as much as necessary - as little as possible' does not constitute an objective piece of advice on which a restorer may base decisions. There have been few scientific studies of potentially suitable cleaning processes (Bauer 1976; Bettembourg and Perrot 1976; Marschner 1986). One of the aims of this project was therefore to shed a little more light in the darkness.
Earlier studies showed that the gel layer can protect the glass beneath it to a certain extent (Scholze 1985; Müller, Torge and Adam 1995). Following on from this, this study examines what cleaning methods can be employed to remove encrustations without damaging this protective layer. A further aim was to examine the effects of the methods adopted in modern practice, both mechanical (bristle brushes, scalpels, glass-fibre brushes, etc.) and chemical (water, organic solutions, special solutions). The suitability of lasers for cleaning glass was tested in the context of a preliminary study (Leissner, Barkhausen, Wissenbach and Fuchs 1994; Leissner, Fuchs, Barkhausen and Wissenbach 1995); however, this area requires much more detailed analysis before practically relevant recommendations can be made.
A dominant theme of the present study was to investigate how extremely firmly fixed encrustations such as those on the choir windows of Erfurt Cathedral can be removed. New methods were developed and tested in the process. Laboratory tests were conducted aimed at discovering if the chemical methods developed in the conservation of wall painting and stonework could be used for removing encrusted layers (see section 3). A selection of the most promising formulae was then tested in the laboratory and on the Erfurt windows themselves (see section 4).
2. New Ways Forward: Cleaning Pastes and Ion-exchangers
2.1. The Formation of Hard Encrustations
A particular problem with cleaning stained glass is the removal of solid layers of corrosion material, such as found on the Erfurt choir windows. These encrustations - known as weathering crust - form from corrosion by-products produced by the reaction between polluted air containing sulphur dioxide and ions leached from the glass itself in the presence of water. Such encrustations on medieval stained glass are generally in the form of calcium sulphate dihydrate (gypsum). Syngenite, a calium calcium sulphate, is also produced as a by-product of these processes, but this tends to disintegrate into calcium sulphate and easily soluble calium combinations.
Calcium sulphate is slightly soluble in water, dissolving in moisture (condensation, rain water) and recrystallizing as the water evaporates; this leads to a constant cycle of dissolving and recrystallization. In the long term, this causes extremely hard encrustations containing soot and dirt to form on the glass. What appear to be the multiple layers of corrosion products on the surfaces of the glass (it is the external ones that are usually affected) are invariably however simply layers of calcium sulphate of varying thickness, consisting of layers of crystalline (or fresh) corrosion products and layers that have become thicker through the recrystallization process (the 'gypsum inter-layer').
During this project a scanning electronic microscope was used to analyze sections of corroded glass with encrustations of this type (see section 4). This clearly showed the complex structure of the layers near the surface of the glass in an alternating sequence, with the gypsum and gel layers breaking through each other on occasions. A gel layer may often be crazed at the interface with the unexposed core glass, though clear-cut boundaries between the various layers were not observed. Thick encrustations of the type described are typically extremely hard and difficult to dissolve in water, making the employment of extremely effective mechanical or chemical measures for cleaning or encrustation removal necessary. However, these measures present further problems, as the gel layer under the corrosion deposits is often soft and vulnerable to damage, particularly mechanical damage.
2.2. Conventional Cleaning Techniques for Hard Encrustations
Numerous examples exist of historical stained glass with scratched and damaged surfaces, demonstrating the dangerous effects of the mechanical methods used for removing corrosion deposits and encrustations. Examples may be found not only from the distant but also the recent past; indeed such methods are sometimes still uses today (fig. 1). The palette of tools ranges from bristle brushes to glass-fibre brushes, scalpels, steel brushes and sand paper. Even careful and controlled use of such tools can cause damage, because the structure of the corroded glass (with hard encrusted layers of corrosion by-products over a soft sensitive gel layer) renders a controlled approach very difficult to achieve. This has been demonstrated clearly by the experiments on model glass (see section 3).
An alternative to mechanical methods is the use of cleaning solutions. Water cannot be used, since gypsum, as already explained, is difficult to dissolve. The solubility of calcium sulphate could be raised by changing the pH-value of the water solution through the addition of acids or bases; however this method no doubt causes damage to the glass and therefore does not enter into consideration. Raising the temperature of the solution in an effort to improve its solvent effect can also not be countenanced in the restoration of stained glass, for practical reasons and on account of the damage it might cause. Organic solvents do not constitute an effective alternative, as they cannot dissolve calcium sulphate.
The effectiveness of water or another solvent as a cleaning agent is clearly improved when the solvent is used in conjunction with a mechanical cleaning method. However, research has shown that the standard process of this type (softening with ethanol or water followed by treatment with a scalpel or glass-fibre brush) can cause damage, because the gel layer is particularly soft and sensitive when in a soaked and dampened state (see section 4).
2.3. New Possibilities
It should be possible in principle to treat historical stained glass more gently with chemical processes that are able to dissolve or recombine calcium sulphate selectively, without affecting the glass or any of its components. There are essentially three possible systems for dissolving gypsum in this way.
Chelating Agents
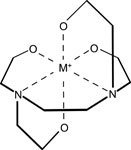
The class of chelating agents includes ethylenediaminetetraacetate (EDTA) and polyphosphate, as well as organic dicarbonic acids or hydroxylic acids (e.g., oxalic acid, citric acid and tartaric acid). The principle on which these chelating agents work is their ability to form complex combinations that are easily soluble in water, with a metal ion (with CaSO4, a calcium ion) functioning as the central ion (see fig. 2).
Ammonium carbonate
In practice, this principle is effected through the conversion of calcium sulphate (CaSO4) into calcium carbonate (CaCO3) using ammonium carbonate ((Nh4)2CO3). The newly formed reaction product CaCO3 is considerably less soluble (0.02g/l) than gypsum (2.02g/l), and must therefore somehow be removed before it forms a chalky film on the surface. The reaction also creates ammonium sulphate ((NH4)SO4); this salt should be removed as completely as is possible, since on the one hand it is a corrosive acidic salt, and on the other is unstable, eventually disintegrating to form ammoniac and sulphuric acid. Any residues of ammonium sulphate can therefore lead to renewed corrosion through acidic action. Cleaning with ammonium carbonate should only therefore be undertaken if these salts can be completely removed (for example by washing the glass after treatment).
Ion-exchanger Resins
Ion-exchangers are organic bodies, mostly in the form of tiny porous spheres, with a multiplicity of surfaces and a water-insoluble three-dimensional structure of complex molecules known as a matrix. Firmly attached to the surface of this artificial-resin matrix are anchor groups to which negative ions are loosely attached; these ions can exchange easily with the ions of a fluid phase. The principle behind ion-exchangers is their ability to exchange ions selectively, whether cations or anions. Different ion-exchangers can be used to achieve different ends. In order to remove calcium sulphate, one can exchange cations such as calcium ions with sodium ions, producing sodium sulphate, which is easily soluble in water. On the other hand, it would also be possible to replace sulphate ions with nitrate or chloride ions (in order to form easily soluble salts), or with carbonate ions (forming calcium carbonate, which is not easily soluble, but stable) (fig. 4).
It should be borne in mind, when selecting a specific ion-exchanger (whether an anion or cation exchanger) and adopting a particular ion-exchanger resin for the purpose, that the combinations formed during the exchange process can have seriously detrimental effects. For example, exchanging cations with hydrogen ions in the presence of sulphates (such as calcium sulphate) invariably leads to the formation of sulphuric acid, which is extremely corrosive. An anion exchange of hydroxide ions for sulphate ions creates a strongly basic medium through the formation of calcium hydroxide, which has a particularly deleterious effect on glass. Before starting the ion-exchange process, one therefore needs to analyse the composition of the corrosion products and the ions to be exchanged (Dorfner 1970; Oeter 1991).
2.4. Practical Experience to Date
Of the methods and processes described above, only chelating agents have been widely employed, particularly in France, where two chelating-agent solutions have been widely used following the work of Bettembourg (Bettembourg 1974; Bettembourg and Perrot 1976).
- Solution A: 10% Na2S2O3 × 5H2O + 5% Na4P2O7 ×10 H2O (a solution of sodium thiosulphate and sodium pyrophosphate)
- Solution B: 3% EDTA + 3% NH4HCO3 (a solution of ethylenediaminetetraacetate and ammonium hydrogencarbonate).
The use of these solutions has been the subject of much controversy, and the damage caused by these very effective solutions has been demonstrated in several studies (Ernsberger 1952; Ferrazini 1977). Further research into the effects of the Bettembourg solutions has been conducted on model glass (see Römich and Fuchs 1992, and section 3.3). In recent years, more knowledge has been gained of dissolving gypsum encrustations from the treatments of corroded stonework and wall-painting surfaces. There is a whole group of systematic studies and reports based on experience, which, because of the similarity in the problems posed, can be applied directly to the cleaning of stained glass (Fritz 1992; Fritz 1996; Oeter 1991). Close parallels can be drawn between the composition and structure of the gypsum and dirt encrustations that can form on the surfaces of lime-bound wall paintings, plaster and calcite stone and those on stained glass surfaces; the way in which these encrustations form is also similar. In all cases one is dealing with the reaction of calcium-based components with the sulphur dioxide found in polluted air, in the presence of moisture.
A significant difference is the fact that plaster and stone are porous, unlike the surfaces of glass, making it easier to use water-based cleaning agents when treating glass. Another difference is that the translucence of glass allows remains of corrosion layers or residues from the cleaning process that have not been cleaned off to be detected more easily with the naked eye. A survey of the literature and an analysis of the practical experience gained in cleaning wall-paintings and stone surfaces therefore provide a good starting point for the development of cleaning processes suitable for treating the stained glass in the choir of Erfurt Cathedral.
2.5. Suggestions for New Cleaning Formulae for Hard Encrustations
Chelating Agents
The chelating agents used in conservation to remove hard-to-dissolve corrosion products (such as the gypsum encrustations described above), react selectively with calcium ions or other metal ions, thereby altering the balance of the solution. During the course of the reaction, the number of ions released into the solution is in proportion to those that become attached to the chelating agents, and successive corrosion layers are dissolved.
A comparison of the reactivities of the most important chelating agents (or the equilibrium constants of the complex reactions they produce) shows that EDTA is the most effective substance for dealing with calcium combinations. The use of weaker chelating agents or substances that have to be used in a more controlled manner is not recommended, as it lengthens the time any water-based solution, a risk in itself, is in contact with the glass. A particular disadvantage of chelating agents is the fact that they react equally with the calcium ions in the gypsum encrustations and the calcium components that are integral to the glass. Every use of chelating agents may therefore constitute an attack on the glass that is still intact and which should be protected above all else.
The reactivity of chelating agents for different ions is very dependent on the pH-value of the solution. The optimal pH-value for the formation of calcium-EDTA-complexes is approximately 12. However, one must bear in mind that alkaline-sensitive glass should not be treated with solutions with a pH-value higher than 8 - though it should also not be treated with solutions with a pH-value below 7, as this severely reduces the speed of the reaction. The pH-value of a water-based solution is therefore more important for the effectiveness of the reaction than its concentration, which means that under suitable conditions concentrations of 1-3% can achieve good results. Of particular interest in this regard is a study made of the reaction speeds of various chelating agents (De Witte and Dupas 1992). This showed that the curve plotted on a graph for reaction speed rose steeply initially, then flattened off swiftly. For practical purposes, this means that application over several hours makes little sense – rather a series of shorter treatments is recommended. EDTA is easily soluble in water. It is normally used in paste or compress form, thickened with cellulose, cellulose-ether or other similar substances. For cleaning corroded glass, EDTA can be used in conjunction with ammonium bicarbonate with a pH-value of 8.
Ammonium Carbonate
Calcium sulphate can be converted into calcium carbonate with ammonium carbonate solutions. In the first stage of the reaction, a large proportion of the calcium sulphate dissolves under the influence of the ammonium carbonate; in the second stage it is converted into calcium carbonate. Of particular importance here is the way the calcium carbonate, a product of the reaction that is not easily soluble, can be fixed in the solution and thereby removed. If this change occurs directly on the surface, becoming in effect almost a topochemical reaction, the effect will hardly register visually, and despite the success of the procedure, the surface remains opaque, covered with a light-obscuring layer. In order to avoid calcium-carbonate residues, or chalk film, ammonium carbonate can be applied as a paste or compress (see above). In addition, other substances that react with calcium can be added, such as carboxymethylcellulose (CMC), Tylose C, or the water-soluble polyacrylic-acid co-polymer Sokalan.
Ion-exchangers
The astonishingly effective and versatile possibilities of ion-exchangers for the restoration or cleaning of corroded glass surfaces have only recently begun to be explored in practice. Their major advantage is that these solutions can be tailored to treat the particular problem posed, whether this is removal of encrustations or salts, rendering chemically neutral, etc. (Burmester, Koller and Kawinski 1990; Oeter 1991; Preis 1996). A further advantage is the strong, water-insoluble structure of the matrix. After the reaction has finished, the tiny artificial-resin spheres, together with the ions attached to them, can be removed. Ion-exchangers are easily 'regenerated': they can be cleaned and re-equipped with the requisite mobile ions.
Methods for dissolving calcium sulphate that could be considered are cation exchange (calcium ions for sodium ions); anion exchange (sulphate ions for nitrate, chloride or carbonate ions); and the simultaneous exchange of cations and anions by means of mixed-bed ion-exchange. From the results of preliminary experiments with a series of various types of ion-exchangers it was apparent that the most successful methods were those that employed anion-exchangers equipped with carbonates in strongly alkaline solutions. Since the ion-exchangers, like all the other cleaning materials already described, can only react with such ions as are present in the water-based solution, the speed of the reaction is dependent on the concentration of calcium ions dissolved in the solution, i.e., on the solubility of calcium sulphate. This can be improved through the addition of ammonium carbonate to the reaction solution.
Experience has shown that the ion-exchange occurs only where there is direct contact between the artificial-resin matrix and the deposits on the surface. For this reason, a longer period of application is not recommended, rather frequent changes of, or restructuring and movement of the poultices is likely to be more effective.
Additives
In order to be applied any one of the three possible cleaning agents in a localized manner, the agent should be applied in the form of thickened paste-like compress. A beechwood cellulose (Arbocel), in various particle sizes, and a carboxymethylcellulose were chosen as thickeners. In addition to its thickening and viscosity-enhancing qualities, the latter also has the ability to combine with calcium carbonate, thereby enhancing the cleaning effect of the process. Other thickening agents and additives with similar qualities that enhanced the effect of the complex-formation process (e.g., the water-soluble polyacrylic-acid co-polymer Sokalan), as well as possible paste holders/applicators, were examined in the same series of preliminary tests.
| Substance (trade name) | Type of material | Effect and area of application |
|---|---|---|
| EDTA | ethylenediaminetetraacetate, chelator | chelating agent used as a solution or in a compress or paste (see fig. 2) |
| Ammonium carbonate | weakly alkaline carbonic-acid salt | reagent for reactions involving precipitation or salt transformation (see fig. 3), used either as a carrier solution for ion-exchangers, or in thickened form as a paste |
| Amberlite IRA416 (produced by Merck) | strongly basic anion-exchanger | resin with potential to carry carbonate, nitrate and acetate |
| Tylose C300 'CMC' (produced by Hoechst) | anionic cellulose-ether, carboxymethylcellulose | thickening agent and co-builder with a high potential for combining with calcium |
| Tylose MH4000 (produced by Hoechst) | cellulose-ether, methylhydroxyethylcellulose | thickening agent with relatively high viscosity used for thickening ammonium-carbonate pastes |
| Sokalan SP5 (produced by BASF) | artificial resin swelling in water, acrylic-resin co-polymer | co-builder with high potential for combining with calcium |
| Agepon (produced by Aqua) | surfactant, mixture of non-ionic and anionic surfactants | wetting agent, used to reduce surface tension and improve wetting |
The range of components selected to ensure or improve the three processes described above - chelating agents, ammonium carbonate and ion-exchangers - is detailed in table 1. The most important formulae tested in this co-operative effort are detailed in table 2. The concentrations of the agents were determined through a series of tests on model glass and laboratory experiments; the concentrations of the additives, particularly the thickening agents, were determined by practical considerations and took the most sensible application method into account. In the first phase of the project the potential effects - both good and bad - of various cleaning pastes and poultices were tested on model glass (see section 3.4); the best formulae were then used in the second phase on samples of original glass from Erfurt (see section 4.4).
| Designation in brief | Principal components | Additives | Advice on production and usage |
|---|---|---|---|
| Ion-exchanger I | Amberlite IRA 416 carrying carbonate | Moistened with demineralized water, Tylose C 300 | Carbonate ions can be attached by treating with a 10% sodium-carbonate solution thickened with Tylose C |
| Ion-exchanger II | Amberlite IRA 416 carrying carbonate | Moistened with a 3% solution of (NH4)2CO3, Tylose C 300 | Carbonate ions can be attached by treating with a 10% sodium-carbonate solution thickened with Tylose C |
| Ion-exchanger III | Amberlite IRA 416 carrying carbonate | Moistened with a 10% solution of (NH4)2CO3, Tylose C 300 | Carbonate ions can be attached by treating with a 10% sodium-carbonate solution thickened with Tylose C |
| Ion-exchanger IV | Amberlite IRA 416 carrying nitrate | Moistened with a 10% solution of (NH4)2NO3, Tylose C 300 | Nitrate ions can be attached by treating with a 10% sodium-nitrate solution thickened with Tylose C |
| (NH4)2CO3 | 10% ammonium carbonate | Tylose MH 4000 (5%) | pH value = 9 |
| (NH4)2CO3 | 5% ammonium carbonate | Tylose MH 4000 (5%) | pH value = 8 |
| (NH4)2CO3 | 2% ammonium carbonate | Tylose MH 4000 (5%) | pH value = 8 |
| (NH4)2CO3 | 5% ammonium carbonate | thickened with whiting | pH value = 9 |
| EDTA | 1% ethylenediaminetetraacetate | thickened with CMC and Arbocel | pH value = 8 applied with (NH4)HCO3 as a buffer |
| EDTA | 3% ethylenediaminetetraacetate | thickened with CMC and Arbocel | pH value = 8 applied with (NH4)HCO3 as a buffer [subscript] |
3. Research into Cleaning Methods Using Model Glass
3.1. Aims and the Programme of Work
Substituting Model Glass for Original Glass
It would not be responsible to undertake comprehensive studies of mechanical and chemical cleaning methods on original glass. It should also be taken into consideration that every piece of original stained glass is unique, and that the validity of comparative conclusions drawn from experiments on an original piece of glass is therefore very limited. The effects of corrosion are difficult to quantify, since the samples cannot be modified for specific methods of analysis. An alternative is therefore to use simulation materials or model glass. Previous studies using calcium-calisilica glass of similar composition to medieval glass have shown that the phenomenological behaviour and corrosion processes of the model glass and original glass are directly comparable (Fuchs, Patzelt and Schmidt 1989).
In the context of this study, selected cleaning methods commonly used in glass conservation will be compared in order to evaluate their damage-causing potential. In this way it should be possible to build up guidelines as to which restoration methods are appropriate for use on real glass, and to identify which should be modified or discarded. The main foci of the programme of work were therefore the simulation of damage to historical stained glass on model glass; the use of selected cleaning techniques on model glass; and the evaluation of how sensitive the treated surfaces were to corrosion. Through comparative analysis of the varying effects of different types of cleaning agents on model glass, the amount of original glass required for preliminary testing can be drastically reduced. However, one must bear in mind that such results may not be 100% transferable in practice.
Preparation of the Model-glass Samples
The chemical composition of the model glass selected for the study should be comparable with that of the medieval glass. However, it should also be borne in mind that a series of tests will only yield useful results if the model glass exhibits measurable corrosion phenomena within a timeframe that is short compared with that of medieval glass. The various types of model glass developed by the Fraunhofer Institute for Silicate Research (Fraunhofer-Institut für Silicatforschung) fulfil these criteria. Model glass types MI, M1.0, M2.0 and MIII were selected for the cleaning experiments described below (see table 3). These types of glass are particularly sensitive to attack from corrosion, making them useful media for laboratory research.
| MI | M1.0 | M2.0 | MIII | |
|---|---|---|---|---|
| SiO2 | 48.0 | 54.2 | 61.2 | 60.0 |
| A12O3 | 1.5 | |||
| P2O3 | 4.0 | |||
| CaO | 15.0 | 17.0 | 23.4 | 25.0 |
| MgO | 3.0 | |||
| Na2O | 3.0 | |||
| K2O | 25.5 | 28.8 | 15.4 | 15.0 |
The majority of the corrosion phenomena observed on historical stained glass can be simulated on these different types of model glass. In order to produce an extended series of samples for these cleaning experiments, accelerated corrosion conditions were created in the laboratory. The fissures often found in the gel layer of original medieval glass can be reproduced by dipping model glass types MI, M1.0 or M2.0 in acid or acidic buffer solutions. The most sensitive type of glass, M1.0, only needs to be exposed to the acid for a period of a few hours. Badly corroded gel layers broke off, thereby creating a multi-layered profile. The gel layers created in model glass were generally thinner (<10μm) than those on the badly corroded medieval originals (up to 150μm).
For these experiments samples with hard encrustations were particularly useful. By weathering model glass in a climate chamber with a controlled temperature and humidity cycle and increased levels of SO2, crystallization layers could be produced within a few days. These crystallized encrustations were porous and easy to remove from the surface. It was not possible under laboratory conditions to simulate automatically the effect of rain that would in turn harden these encrustations. For this reason the weathering process in the climate chamber was interrupted several times, the surfaces washed briefly with water, and the samples returned to the climate chamber. The hard encrustations produced in this way proved suitable for the experiments. However, it was not possible to simulate on model glass the truly hardened encrustations that occur on medieval glass, created by the lengthy process of dissolving and recrystallization of corrosion products over many years (such as found on the choir windows at Erfurt, see section 4).
Further experiments with an exsiccator, whereby the glass was exposed to particularly high concentrations of SO2 in gas state with the aid of a special solution, produced similar results. As with the samples weathered in the climate chamber, after the encrustations had been cleaned off, the surface of the glass exhibited the fissure pattern typically found in gel layers damaged by corrosion. Examples of some of the different types of damage to the model glass are shown in fig. 5.
-

- Fig. 5. Scanning-electron-microscope images of corrosion phenomena that occur on medieval glass simulation on model glass (magnification given below each image): (a) and (b) surface and cross-section of model glass MI after an hour's exposure to a 5% hydrochloric-acid solution; (c) and (d) surface and cross-section of model glass M1.0 after two weeks in the climate chamber, into which SO2 was introduced occasionally.
Pitting, a type of corrosion that occurs on medieval glass, could only be simulated by weathering model glass in non-laboratory conditions. Under these conditions, the particularly sensitive glass types MI and M1.0 developed less regular fissure patterns in the gel layer than model glass treated in the laboratory. With exposure periods of more than a year, concentric cracks could be observed to deepen, leading to the localized breaking off of fragments from the surface; this could be denoted an early stage of pitting.
Methods of Analysis
Deposits of crystals and cracks in the gel layer of the pre-damaged and cleaned samples were examined using light microscopy, with a magnification factor of 200. Selected samples were also examined with a scanning electron microscope, with magnification factors of up to 2000. The progress of corrosion to the model glass samples was tracked quantitively using the process developed by the Fraunhofer Institute for Silicate Research for the evaluation of glass sensors (see Protective Glazing). Infra-red spectra were measured in transmission and the increase in the hydroxyl group absorption bands tracked at 3300cm-1 (the 'E-value'). In this spectrum, the water content of the gel layer, depleted of potassium and calcium, as well as that of the by-products of weathering, such as syngenite and gypsum, can be registered.
It is recommended that E-values not be regarded as absolute, but that fluctuations in them be interpreted as ΔE-values. Concrete examples of the use of infra-red spectroscopy for the evaluation of cleaning experiments are detailed in what follows.
3.2. Mechanical Cleaning Methods
Choice of Cleaning Tools
The mechanical cleaning tools studied ranged from bristle brushes, glass-fibre brushes and scalpels to steel wool, wire brushes and sand paper, in order to reveal the gamut of possible damage resulting from mechanical cleaning. Several series of tests were undertaken in order to clarify and research the nature of the actual damage caused, as well as the effects of damage residues on the progress of corrosion. The particle-beam abrasive (the 'airbrasive') used in Great Britain is a special case; results of a study of this process are detailed in the final report (Römich and Fuchs 1992; Römich and Fuchs 1996). For the purposes of this study, three examples were chosen.
Research into Possible Damage through Mechanical Cleaning
Model glass type MI, on which (as noted above) a porous encrustation formed in the climate chamber, was relatively simple to clean by mechanical methods. Following the removal of the encrustation, it was possible to see the fine fissures of the gel layer using a light microscope. This damage had been inflicted in the preliminary treatment, and was the same on all the samples. The extent of the damage to the surface of the gel layer can be established by the type of damage and the frequency with which this occurs.
Cleaning with steel brushes and sandpaper led to serious damage to the surface, with deep scratches and furrows. Glass-fibre brushes, bristle brushes and scalpels left localized fine scratches; occasionally fragments of the gel layer had flaked off, and strips of residues from the cleaning process were observed. Great differences were noted in the results, depending on whether hard, blunt or soft brushes were used, and the angle at which the scalpel blade was applied. Cleaning with steel wool resulted in a smooth surface, though it is impossible to guarantee that parts of the surface were not also removed in the process; for this reason this method is not recommended for historical stained glass.
Examples of the actual damage caused to surfaces of the model glass after mechanical cleaning are shown in fig. 6. The long-term effect of mechanical damage to the surface of the glass could be simulated by treating the cleaned samples to a further period of corrosion in the climate chamber. This subsequent weathering produced a crystalline crust on the samples, the problem being clearly worse in places where there were traces of scratches.
Similar cleaning tests were carried out on model-glass sample of types M2.0 and MIII. Glass that is more weather resistant on account of its chemical composition also tended to be less damaged by mechanical cleaning. However, in places where deep scratches had been caused, the effects of corrosion were observed to be exacerbated after further weathering.
Research into the Ease with which Mechanical Cleaning Tools Can Be Used
Meaningful statements on the damage-causing potential of specific mechanical cleaning methods can only be made if various pre-corroded samples are cleaned by different people in separate experiments. For this purpose several series of experiments were undertaken within the context of workshops at the Fraunhofer Institute for Silicate Research. Ten conservators from different studios were invited to collaborate with scientists in the laboratory in the cleaning of model glass; to evaluate the results themselves; and then to discuss them.
Over 400 model-glass samples were used, to achieve the broadest possible statistical basis for the analysis of localized damage to sensitive glass and its relationship to the use of particular cleaning tools. While heavy damage to the surfaces was easy to determine, for example when sand paper was used, it was not possible to differentiate between the damage caused by glass-fibre brushes, bristle brushes and scalpels. Some of the participants were able to clean corrosion with little or no damage to the surface using a scalpel, while others needed the bristle brush to achieve the same success. The amount of damage to the gel layer depended more on the way in which a particular tool was used - in other words the conservator’s personal technique - than on the choice of tool.
For this reason it will not be possible in the future, as now, to establish general guidelines for the choice of cleaning method: the skill of the conservator will be more important in determining the success or failure of a restoration project. Scientific studies may supply basic principles, but these must be converted into practice by the conservator.
Research into the Effects of Residues
When cleaning historical stained glass it is imperative that damage to the gel layer be avoided. The extent to which crystalline encrustations should be removed or thinned, from a scientific point of view, was the subject of a further series of experiments.
Samples of model glass type M2.0 were pre-corroded to create a porous layer of corrosion products. After cleaning with various methods the samples were corroded again, along with uncleaned samples for comparison. The progress of corrosion in all the samples was tracked with infra-red spectroscopy. In this case, the ΔE-values are a measure for the sensitization of the glass surface through cleaning.
In fig. 7 the ΔE-values of the samples are shown after the removal of the porous encrustation. The highest ΔE-values were measured with samples cleaned with sandpaper, with greater degrees of corrosion demonstrated where the gel layer was damaged. The samples that had been cleaned to protect them were all relatively similar, with much lower values. The relatively high values for the uncleaned samples showed that the porous encrustation accelerated the corrosion process. In this case, careful cleaning slowed the corrosion process on sensitive glass.
This effect was less pronounced after cleaning hard, non-porous encrustations on M2.0 model glass, and did not occur at all in the series of experiments on MIII model glass. The capacity of the crystalline encrustations to absorb water, which in turn depends on their porosity and composition, is the decisive factor in the progress of corrosion to glass. This factor is also a symptom of the difficulties faced in attempting to formulate general guidelines for the cleaning of historical stained glass, with the huge variety of glass types involved, each with their own characteristic corrosion profiles and encrustations.
3.3. Chemical Cleaning Techniques
Choice of Cleaning Method
The principal foci of the programme of work on chemical cleaning methods were water-based solutions (e.g., water, oxalic acids, ammonium-carbonate solutions) and organic solutions (toluene, ethanol, acetone), whose effects on badly corroded stained glass were tested. Two examples of experiments from this programme are described in what follows. The effectiveness and damage-causing potential of chemical scouring pastes, solvent poultices, and solvent pastes generally used in conservation practice were tested by the conservators of the stained glass studio at Cologne Cathedral (Jägers and Brinkmann 1999).
Cleaning with Water-based Solutions with Varying pH-values
Water is an extremely versatile solvent, much used in conservation. The action of water as a solvent can be optimized for a particular purpose through the use of additives (buffers, surfactants, chelating agents, salts, thickeners). The most important way of optimizing the action of water for such a purpose is to change the pH-value. Many substances that are not soluble in pH-neutral conditions can be dissolved in acidic and base conditions, since acids and bases can accelerate the break-down process.
Acids and alkalis were much used in previous centuries for the cleaning of stained glass, and in the 1970s there were still experiments with 'acid polishing' on historical stained glass (application of a 40% acid solution) (Bauer 1976). The pH-value of water-based solutions governs the action of such solutions on glass. In acidic conditions, this reaction leads to a leaching of network modifiers (e.g., K and Ca) and the formation of a gel layer, whereas under alkaline conditions the structure of the glass itself comes under attack, and the glass dissolves. Additive-free water also attacks glass, depending on the glass’s chemical composition and stability.
Leaching experiments on model glass were carried out by the Fraunhofer Institute for Silicate Research in order to compare the effectiveness of basic and acidic cleaning solutions. In a series of experiments, samples of MI glass were immersed in 5% solutions of oxalic acid, tartaric acid, hydrochloric acid and EDTA, as well as in water with and without surfactants, and in a 3% ammoniac solution. One sample had oxalic-acid paste thickened with whiting brushed onto it. The samples were examined after an hour, and subsequently treated for a further 16 hours.
Samples immersed in strongly acidic acid solutions with a pH-value of 1 (hydrochloric acid, oxalic acid, tartaric acid) and in an EDTA solution with a pH-value of 4 exhibited an extremely dense network of fissures in the gel layer. Oxalic-acid paste (with a pH-value of 4) had a less aggressive effect than oxalic-acid solution. Neutral solutions (water) or base solutions (ammoniac) caused short cracks or spots of deposit to form. Typical scanning-electron microscopy pictures are shown in fig. 8. The pH-value of the strongly acidic solutions barely changed, but that of the EDTA solution rose from 4 to 6. This was caused in the weakly acidic solutions by the leaching of ions from the samples (with H+ being exchanged for Ca2+ or K+). The pH-value of the strongly basic ammoniac solution (NH3) fell from 12 to 11. The process of dissolving the glass used up all the OH--ions (Scholze 1988).
The largest reduction in mass was observed with the sample immersed in hydrochloric acid (0.210g), followed by the samples treated with oxalic acids (0.052g) and EDTA (0.046g). The reduction in mass was caused by the exchange of heavy earth-metal alkali ions (Ca 40g/mol) and alkali ions (K 39g/mol) with H+ or H3O+ (19g/mol). Samples immersed in NH3 showed little reduction in mass (0.002g). The process of dissolving the glass was much slower in basic conditions than the leaching of the glass under acidic conditions.
The varying reactions exhibited by the model-glass samples under acidic and alkaline conditions could be assessed on the basis of this series of experiments. The process of leaching in acidic conditions led to serious damage to the glass. Acids should therefore not be used in principle for the conservation of glass. A possible exception might be the use of oxalic-acid solutions and pastes for the cleaning of eighteenth- and nineteenth-century glass; however the glass should only be exposed for short periods and should be suitably treated afterwards by washing. Slightly alkaline solutions or water could also be used for similarly short periods to clean sensitive glass; however, exposure times should be kept to an absolute minimum, as there is a danger not only that the surface of the glass may change, but that the glass itself may begin to dissolve.
Cleaning with Organic Solutions
Organic solutions are used in many areas of conservation to remove organic dirt deposits, e.g., oil and fat, old lacquer and coatings, colours and adhesives (Ashley-Smith 1987; Horie 1992). The following classes of substance were considered.
- nonpolar hydrocarbons (e.g., hexane)
- aromatic hydrocarbons (e.g., toluene)
- halogenated hydrocarbons (e.g., methylene chloride)
- polar oxygen-rich hydrocarbons (e.g., alcohol, acetone, ethyl acetate).
The following points should be considered when selecting a solvent for a specific cleaning task.
- The surface tension of the solvent should allow good interaction with the sample.
- For porous items, the solvent should be prevented from dissolving the surface dirt and depositing it in corrosion pits before it can be removed.
- The steam pressure of the solvent should be low enough that the surface dries relatively quickly.
- At the same time, the cleaning medium should not be too volatile and should not evaporate until the cleaning process is complete. In many cases mixtures of solvents can be employed to achieve a compromise between the intended effect of the solution and the time it takes to dry.
- The toxicity of the solvent must be taken into account.
The polarity of the solvent determines how the solution behaves and therefore its appropriateness for specific cleaning problems. In the conservation of historical stained glass windows, organic substances such as acetone, toluene, dimethyl sulfoxide, vinegar-ester (acetic acid ethylester) or alcohol (spirit) have been used to remove the remains of putty, adhesives or substances formerly used for conservation. Such organic solvents are not suitable for the removal of inorganic encrustations such as gypsum or syngenite. As an inorganic material, glass can be cleaned with organic solvents without fear of damage, since interaction is expected to be minimal. However, the use of nonpolar solvents on badly corroded glass with thick gel layers can lead to loss of water from the gel layer and cause the cleaning process to have uncontrolled consequences such as water loss, shrinkage of the gel layer, and flaking. Since polar solvents (e.g., acetone, alcohol, dimethyl sulfoxide) when bought include a certain percentage of water or attract water during application, they can damage sensitive glass.
Some research has been undertaken previously on the effects of organic solvents on glass surfaces (Marschner 1986). However, their effect on original glass could not be quantified, despite the use of microscopy and measurements of the rawness of the surface. Since only minimal interaction was to be expected, the experiments at the Fraunhofer Institute for Silicate Research were carried out on comparable pre-corroded model-glass samples and the effects tracked with infra-red spectroscopy.
The next series of experiments looked at the removal of water from the gel layer with organic solvents. This was carried out on MI-glass samples that had been leached with an acidic buffer solution, producing a badly cracked gel layer, though without crystalline deposits. The samples were immersed for several hours in acetone, toluene, dimethyl sulfoxide, or vinegar-ester. Directly after being removed from the solvent, the water content of the gel layer was established by means of infra-red spectroscopy and tracked during its subsequent exposure to air, with spot measurements every few minutes.
It was shown that the treatment of the model-glass samples with organic solutions led to the loss of water from the gel layer. The samples treated with acetone dried very quickly (within a minute), whereas dimethyl sulfoxide (boiling point 189°C) was much less volatile than acetone (boiling point 56°C) and could still be detected for a long time after the model-glass samples had been removed from the solvent bath. The thicker the gel layer, the greater was the loss of water. The water content of the gel layer returned to normal after exposure to moisture in the air, but much more quickly after treatment with acetone than with dimethyl sulfoxide.
Original medieval glass has thicker gel layers and more deeply rutted surfaces than model glass, and the processes of water absorption and water loss are probably slower. It must be borne in mind that when using highly volatile solutions such as acetone the speed of the processes of water absorption and water loss leads to extra stress to the gel layer of the glass, even when it is wiped clean after treatment. By contrast dimethyl sulfoxide dries so slowly that extra cleaning with ethanol is recommended to remove as much as possible of any dimethyl sulfoxide remaining on the surface. The behaviour of the other solvents studied (toluene, vinegar-ester, ethanol) lay between the extremes of acetone and dimethyl sulfoxide.
The model glass appeared to have suffered no damage when examined using light microscopy, but the spot-measurements using infra-red spectroscopy on the corroded glass after immersion in the solvent bath showed that every cleaning episode affected the water content of the gel layer. In our current state of knowledge, these experiments confirm that organic solvents can be used for cleaning of historic stained glass, although generally speaking, as ever, unnecessary treatment should be avoided.
3.4. Cleaning Pastes and Ion-exchangers
As already described in detail in section 2, new formulae for cleaning hard encrustations on glass have been developed that had previously proved their worth in solving similar cleaning problems in the conservation of stone and wall paintings. New cleaning solutions were tested on model glass in various stages, and the many series of experiments can only be summarized here.
Following on from the experiments described in section 3.3 concerning the effects of water-based solutions with varying pH-values, the effects of the two Bettembourg formulae, A (Na2S2O3/Na2P2O4) and B (EDTA/NH4HCO3), as well as those of EDTA and ammonium carbonate were researched and compared with those of water. The damaging effect of EDTA on uncorroded model glass was described in section 3.3. The model-glass samples with thin gel layers used in this series of experiments showed that the gel layer could function as a barrier against 1%- and 3%-solutions of EDTA (pH-value 4.5), as has also been shown in previous studies (Müller, Torge, Kruschke and Adam 1997). The loss of calcium from the core glass is only reduced by this barrier effect, not prevented. The ammonium-carbonate solutions (7% and 10%, pH-value 9) appeared much less damaging than the EDTA solutions, but possessed nevertheless a higher damage potential than water overall.
In a second stage of the programme, poultices steeped in EDTA (with CMC and Arbocel added as thickeners) were tested and compared with those steeped in ammonium carbonate and the first ion-exchangers (IRA 416, carbonate ion-exchanger). As the results of the second stage were so promising, various other ion-exchangers (Amberlyst A-21, Amberlite IRA 416 and Lewatit 500) were tested and compared with ammonium-carbonate poultices in a third stage. The aim of the fourth stage was to compare EDTA-poultices and ion-exchangers. The damage potential of treatment with ion-exchangers for 100 hours equated to that of treatment with EDTA for 12 hours (tested on MI model glass with a thin gel layer).
Experiments on original glass (see section 4.4) showed that the strongly basic ion-exchanger IRA 416, which was equipped with carbonate ions, tended to form a thin chalky film on the surface, an undesirable side effect. The fifth stage therefore looked at developing new formulae for ion-exchangers with various ion-exchanging permutations that did not lead to a deposit of chalk.
However, ion-exchangers equipped with nitrate and acetate ions were not as successful at cleaning hard encrustations from model glass MI as those equipped with carbonate ions. Mixed-bed ion-exchangers and fluid ion-exchangers did not constitute suitable alternatives to IRA 416. Fig. 9 shows a model-glass sample covered in ion-exchanger spheres.
The effects of the most promising variants of ion-exchanger were tested in a further series of experiments. New formulae were included in this series in an attempt to reduce the side-effect of chalk deposition described above. Attention was given to how many times the ion-exchangers had to be changed in order to be effective, by analyzing the levels of sulphate ions released after each change.
In the last series of experiments the effectiveness of a total of 6 ion-exchanger formulae (Amberlite IRA 146, equipped with carbonate ions or nitrate ions, with various additives) was compared with that of ammonium-carbonate solutions and pastes. The technical difficulties experienced in using ion-exchangers on original glass meant that in practice conservators preferred ammonium-carbonate poultices. The results on model-glass samples supported the observation that ammonium-carbonate pastes should be considered as alternatives to ion-exchangers.
3.5. Summary
The approach above described showed that experiments with model glass are important, if one is testing the effect of new cleaning formulae before trying them on original glass. Because results must be analyzed comparatively, a staged approach is necessary, comparing the most promising variants with new formulae at subsequent stages. Of the 35 or so other cleaning pastes and ion-exchangers that were applied to model glass for various lengths of time, very few were tested further on original glass. These selected cleaning media have already been detailed in table 2 (see section 2). The effect on original glass from Erfurt will be described in section 4.
4. Research into Cleaning Methods on Original Glass
4.1. The Programme of Work and Preparation of Samples
The aim of this research was to evaluate the effect of cleaning processes on original glass. The scanning electron microscope and electron-ray micro-analysis are suitable tools for analyzing the changes that occur in the core glass, the gel layer, and corrosion by-product layer in sections from samples of original glass. Both tools had to be optimized for this particular task. The programme of research with samples of original glass centred on the development of a suitable technique for preparing glass for electron-ray micro-analysis; the assessment of mechanical cleaning methods; and the assessment of chemical cleaning methods.
In order to check the effectiveness of the various cleaning measures, pieces of glass approximately 10–20cm2 should be removed from the leading of the panels being treated, cleaned, and replaced in the panel after examination. In every case, the cleaning of samples should be undertaken by the same conservator who works on the whole panel, in order to be able to judge the conservator’s personal style in the handling of the various tools used.
By dividing a sample panel into several zones, each treated with different tools and varying degrees of intensity (bearing in mind that the sample must be cleaned evenly, right to the edges), it is possible to compare the various treatments with one another. The edges of the sample should be perfectly ground and polished as a prerequisite for assessing the effectiveness of a cleaning method and its damage-causing potential, and must be ground and polished in such a way as to ensure that the surface layers are intact up to the edge. This is a major difficulty with this method, as such faultless preparation requires considerable experience.
In the course of the project, the collaboration between several workshops meant that high levels of certainty could be achieved when making assessments. (The results of this collaboration are outlined in what follows by means of a few examples.) However, it was not possible to rectify fully the inadequacies in the preparation of the samples. In some cases, even transporting the samples was enough to damage the gel and corrosion layers. The pressure exerted during grinding and polishing increased the tendency of the gel layer to shed fragments. Where the structure of the sample was loose, even bedding the sample in resin did not help. With these limitations in the research method in mind, a realistic approach must be adopted, such that any assessment of cleaning methods derived from the measurements taken must not be considered absolute, but can support a conservator in any decision he has to make based on his experience with a high degree of probability.
In order to facilitate the assessment of these cleaning experiments, the methods were altered and expanded in some cases. The polishing of the edge of one suitable sample was undertaken before cleaning, and in addition pyramid-shaped impressions were made in the polished core glass near the gel layer with a Vickers-diamond, under the microscope (fig. 10). The exact point from which measurements are taken can be easily identified with this marking, so that an accurate assessment can be made, by comparing the condition of the surface before and after cleaning (figs 10 and 11). This also allows any changes in structure, which can occur with chemical and mechanical cleaning, to be determined very easily by direct comparison.
4.2. Mechanical Cleaning Methods
Within the framework of the project, the possibility of research using electron-ray micro-analysis was offered to several glass workshops. Systematic preliminary research on the evaluation of mechanical cleaning methods was undertaken in the course of the following conservation projects.
- Erfurt Cathedral (cathedral studios in Cologne and Erfurt)
- Cologne Cathedral (cathedral studio in Cologne)
- Church of St Catherine, Oppenheim (Oidtmann Workshop, Linnich)
- Church St Stephen, Constance (Saile Workshop, Stuttgart)
- Church at Kloster Neuendorf (Wilde Workshop, Bellingen)
- Church of Our Lady, Oberwesel (Binsfeld Workshop, Trier)
- Lincoln Cathedral (cathedral studio in Cologne, workshop in Lincoln)
Separate projects along similar lines (Müller and Torge 1998) supported by the Deutsche Bundesstiftung Umwelt allowed further examples to be studied at
- Stendal Cathedral (Lehmann Workshop, Berlin)
- Havelberg Cathedral (Wilde Workshop, Berlin)
- Halberstadt Cathedral (Losert Workshop, Halberstadt).
The aim was to analyze various cleaning methods and varying degrees of intensity of treatment. Because of the number of objects studied and therefore of glass types, there was a correspondingly wide variety in the condition of the glass resulting from corrosion. According to information supplied by the workshops, the following tools and mechanical cleaning methods were used: sponges, bristle brushes, glass-fibre brushes, scalpels, and an airbrasive tool.
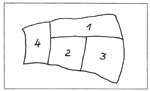
An original sample from the medieval glass of the Church of St Catherine in Oppenheim will serve as an example of how cleaning can be researched. The schematically sketched sample shown in fig. 12 (from rose window south III, dating to 1332–33) was cleaned in different ways in the zones numbered 1 to 4:
- 1 with a bristle brush;
- 2 with a scalpel;
- 3 with a scalpel and glass-fibre brush;
- 4 with a scalpel and glass-fibre brush after the encrustation layer had been moistened for an hour with spirit.
The transparency improved after cleaning from zone to zone. Figs 12–20 show clear differences in the degree to which the encrustation was removed.
Sample zone 1, cleaned with a bristle brush, had a gel layer between 20μm and 60μm thick (fig. 14). There was a mixed external layer of corrosion products (gypsum) and gel layer fragments across the whole surface, approximately 100μm thick; this was thinned out in a few places by the bristle brush. The sample zone cleaned with the scalpel (cleaning method 2) also had a gel layer throughout. Parts of the corrosion by-product layer remained in places, though in other places the layer was extensively reduced. Damage to the core glass need not be feared, as it was completely covered by the gel layer. With cleaning method 3 the gel layer was damaged throughout. Softening the surface encrustation with spirit and subsequently removing the corrosion encrustation with a scalpel and glass-fibre brush (cleaning method 4) led to the total loss of the gel layer. Traces of this cleaning intervention were also left in the core glass, which was undamaged by the other methods. As a result, this cleaning method can be expected to cause serious damage to the original substance of the glass. This is hardly surprising: careful use of a soft bristle brush and scalpel in the hands of an experienced restorer did not cause damage to the gel layer; however, the use of the hard glass-fibre brush (the hardness of the glass being considerably greater then that of the gel layer) is potentially very damaging, particularly if the glass has been moistened first. Further examples of the influence of the composition of glass on the way in which the gel layer and corrosion by-product layer develop and change, and associated possible cleaning methods are detailed in the final report (Müller, Torge, Kruschke and Adam 1997).
If the conservator, in agreement with a monuments' inspector, has decided to remove corrosion products from medieval stained glass, the next stage is the development of a strategy that takes all possible risks into account and is specific to that glass. It goes without saying that the paint layers must not be damaged in any way. This applies also to residues of paint layers on external surfaces, which are often very difficult to identify under layers of dirt and corrosion products. The main aim must be to achieve a consistent optical effect, without extreme differences in transparency, and to minimize the progress of weathering in future. The preservation of the gel layer under the corrosion layers is all important for this.
The methods of analysis used in the examples outlined above were also employed to assess the potential usefulness of mechanical techniques on the hard encrustations on the surfaces of the glass in Erfurt Cathedral choir. The use of bristle brushes and scalpels only achieved a minimal reduction in the corrosion crust, and therefore little improvement in the transparency of the samples. Intensive cleaning with a scalpel improved transparency, but at the cost of complete loss of the gel layer (figs 21 and 22). These methods were therefore not used on the Erfurt material.
4.3. Chemical Cleaning Processes
It has already been noted that the corrosion products on medieval glass surfaces can vary greatly. Cleaning using traditional methods (as outlined in section 2.2) should only be undertaken on relatively loose encrustations. The removal of hard encrustations by these methods would be dangerous, as it could damage the gel layer and the core glass beneath. For these reasons, chemical cleaning methods have been employed in the past.
Hydrochloric acid was used even in the 1950s to improve the transparency of the medieval glass panels from Neukloster (Mansfeld 1959) (figs 23 and 24). The effects and consequences of this treatment can now be studied after almost half a century, in the light of our present state of knowledge (Müller, Torge, Adam and Kocher 1996). The structure of the surface deposits is not typical of the types of corrosion observed to occur with normal atmospheric pollution. As well as uniform, white layers of fine crystals on the external face of the glass, the internal face was seen to have lumpy swellings, with the surface layers flaking off in places (fig. 23). The scanning-electron-microscope image of a cross-section of a segment of glass shows clearly the broad areas where the glass has been transformed by the hydrochloric-acid treatment, with layers of corrosion products (fig. 24). The hydrochloric acid has caused changes in the structure and thickness of the gel layer, and the original substance of the glass has also been damaged. It is therefore also necessary to test chemical cleaning methods on segments of glass before trying them on large areas of original glass.
This research was particularly interesting, because of the use of EDTA (see section 2.2), though an effective solvent for gypsum, poses the risk that calcium ions are removed from the glass. The use of EDTA poultices was tested on samples from a panel from sVI in Erfurt cathedral (table 2). Each of the samples taken from the panel for the different cleaning experiments was ground, polished and marked a Vickers diamond along one edge. The hard encrustations were partially removed with a 3% EDTA solution (using a buffer solution of ammonium hydrogencarbonate, pH-value 8) thickened with Arbocel to the required paste consistency. The application time was 12 hours, with the compress changed every 4 hours. The EDTA residues were subsequently completely removed by rinsing with distilled water.
The condition of the gel layers at the marked test areas was recorded by electron-ray micro-analysis before and after cleaning (figs 25 and 26). (This marking of the polished surface of the edge allowed the state of the gel layer to be assessed at exactly the same test areas each time.) The partial removal of the surface corrosion layer did not affect the gel layer, its structure remaining identical. No damage to the glass through the effects of cleaning poultices could be identified for this particular treatment (figs 25 and 26).
EDTA solutions dissolved the corrosion by-product layer much more quickly than any of the other chemical cleaning method (see the next section). However, it is not possible to judge how far this method can be applied in workshop practice with the same results. The smallest changes in the pH-values of the poultices or a lengthened period of treatment for a particular case can lead to damage to the medieval glass. A sufficiently thick, protective gel layer must exist before treatment with EDTA can be countenanced, which the conservator cannot establish without undertaking scientific research.
4.4. Cleaning Pastes and Ion-exchangers
The research on model glass described in section 3 is particularly useful when a large variety of methods is available and the methods need to be compared with one another. Model glass can be produced to an exact and constant specification, and this facilitates extended series of experiments.
The treatment of the glass in these experiments does not have parameters that can be transferred directly into practice however: the complexity of the weathering processes on original glass is not directly comparable with those achieved in the climate chamber on model-glass samples. The second stage of the assessment process should involve the evaluation of the various cleaning methods under real conditions. In the framework of this study, the various cleaning methods optimized during experiments with model glass were applied to representative segments of glass from panel sVI 5a from Erfurt Cathedral, in order to establish the minimum necessary and maximum possible lengths of treatment under scientific conditions (table 4, samples P1-P5).
| P1 | P1 | P2 | P2 | P3 | P3 | P4 | P4 | P5 | P5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| glass | gel layer | glass | gel layer | glass | gel layer | glass | gel layer | glass | gel layer | |
| Na2O | 1.9 | - | - | - | - | - | - | - | - | - |
| MgO | 4.1 | - | 3.0 | 1.7 | 3.0 | 2.1 | 2.7 | 1.2 | 3.2 | 0.2 |
| A12O3 | 3.1 | 4.6 | 0.6 | 0.8 | 1.0 | 2.1 | 1.4 | 2.3 | 1.2 | 1.6 |
| SiO2 | 50.5 | 76.9 | 50.4 | 82.3 | 49.5 | 84.3 | 49.4 | 85.5 | 49.6 | 80.7 |
| P2O5 | 5.5 | 3.3 | 0.8 | 0.8 | 0.6 | 2.1 | 1.3 | 1.2 | 1.2 | 1.9 |
| SO3 | - | 9.6 | 0.9 | 6.0 | 0.3 | 1.9 | 0.6 | 2.3 | 0.7 | 9.4 |
| Cl | 0.1 | - | - | 1.1 | - | 1.1 | - | 0.2 | - | 0.2 |
| K2O | 7.6 | 1.3 | 25.3 | 1.0 | 22.3 | 0.8 | 24.2 | 0.2 | 22.3 | 0.5 |
| CaO | 25.3 | 3.1 | 18.2 | 4.5 | 22.4 | 4.2 | 18.5 | 2.0 | 22.9 | 3.3 |
| MnO | 1.5 | 0.3 | 0.8 | 1.4 | 0.7 | 0.8 | 0.8 | 0.7 | 0.8 | 0.7 |
| Fe2O3 | 0.4 | 0.9 | - | - | 0.2 | 0.6 | 2.0 | 3.7 | 0.2 | 0.7 |
| BaO | - | - | - | 0.3 | - | - | - | 0.4 | - | - |
| PbO | - | - | - | - | - | - | 0.2 | - | - | 1.6 |
| CuO | - | - | - | 0.3 | - | - | - | - | - | - |
The corrosion-product layers on the original samples differed in thickness by up to several hundred μm, and the gel layers varied in depth from 20μm to 40μm. The results of analysis of the composition of the glass and gel layer and of individual components of the encrustation are shown in tables 4 and 5.
| Measurement point | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| MgO | 0.2 | - | 0.4 | 18.5 | - |
| A12O3 | 1.2 | - | 14.2 | - | - |
| SiO2 | 52.3 | 0.8 | 49.3 | 2.9 | - |
| P2O5 | 3.1 | - | - | - | - |
| SO3 | 25.5 | 68.0 | 8.7 | 1.5 | 22.5 |
| C1 | 2.1 | - | - | - | - |
| K2O | 0.7 | 0.7 | 11.5 | 0.5 | 2.7 |
| CaO | 7.7 | 30.5 | 13.0 | 29.2 | 22.3 |
| CuO | 2.4 | - | 1.0 | - | 26.0 |
| components include | gel layer and gypsum | gypsum | feldspars | calcite | gypsum |
As always, measurements of the corrosion-product layers were made by electron-ray micro-analysis at marked points. Since the corrosion-product layers had not built up in a consistent manner, their composition was haphazard. In no way did they represent a norm for the way in which these encrustations build up, and they produced evidence for a whole variety of crystalline components. The presence of calcite and feldspar as well as gypsum can be important when considering treatment with an anion-exchanger.
Attempts were made to remove the encrustations partially from the medieval glass at Erfurt after evaluating research into the effect of one particular optimized ion-exchanger on model glass (Amberlite IRA 416, equipped with carbonate, moistened with 10% ammonium-carbonate solution and thickened with Tylose C300). The poultices were changed every two hours during the day and covered with foil at night to prevent them drying out. The cleaning effect achieved with this treatment with ion-exchangers differed greatly from sample to sample. After treatment for 33 hours, the corrosion encrustations on one segment of sample P5 had been so reduced that the transparency of the glass was much improved (figs 27 and 28). The corrosion encrustation was not completely removed, but corrosion pits under it were exposed. The state of the gel layer in marked test areas was documented before and after the chemical treatment with a microprobe (figs 29 and 30). The gel layer was not altered by this partial removal of the corrosion encrustation from the surface, and the structure of the gel layer remained unaltered. No damage could be shown to have been caused to the glass by the cleaning poultices.
Neither the lengthening of the treatment period to 110 hours nor the variation of the additives (Agepon, Sokalan) had any measurable effect on the encrustations on samples P2 and P3 (fig. 31). The reasons for this imperviousness to solvents could have been the mineral composition of the encrustation. The higher percentage of calcite (calcium carbonate) in the corrosion encrustations on samples P2 and P3 could have neutralized the effect of the cleaning poultices, as the latter were optimized to exchange sulphate ions for carbonate ions. In such cases, an attempt can be made to dissolve the corrosion layer with EDTA (see section 4.3). In this case, the application of EDTA poultices for 12 hours with changes every 4 hours achieved a similar result similar to that achieved by the particular type of ion-exchanger used (figs 28 and 32). Hard corrosion layers can be removed much more quickly with EDTA solution than with ion-exchanger poultices, and the slightly increased risk to the glass this presents appears to be within acceptable limits. After 12 hours of treatment there were no recognizable changes to the gel layers (figs 25 and 26).
After these tests on single samples removed from the leading, the next step was to test segments of glass from the test panels from Erfurt Cathedral (sV 4c, sV 4d, and nVII 3c) still in their original leading. This series of tests consisted in applying poultices with the ion-exchanger Amberlite IRA 416 with 3-10% ammonium-carbonate solution, both with and without the addition of Tylose, and pure ammonium-carbonate poultices thickened with Tylose C300 or MH 4000 and Arbocel (see section 3.4 and table 2).
There was a whole range of questions to be answered regarding application in practice; suitable methods for applying and removing the cleaning pastes had to be tested; and the length of application of the poultices and the optimal number of poultice changes needed to be determined (fig. 33). Problems of sealing the edges needed to be addressed, and the consistency of the poultices needed to be adjusted so that the inner face of the glass did not come into contact with the cleaning solution. Cyclododekan, a volatile binder much used in other areas of conservation, was used to seal the lead cames (Hangleiter, Jägers and Jägers 1995; Hiby 1997). This was a basic requirement before poultices were applied to the test areas. This material, which even in its solid state is slightly volatile and wax-like, prevents the compress fluids trickling through and affecting the layers of black stained glass paint on the inner face of the glass. Furthermore the poultices must be covered for the whole length of the treatment, to avoid their drying out prematurely.
-

- Fig. 33. Working with ion-exchangers and cleaning compresses at the glass restoration workshop in Erfurt Cathedral: (a) two panels with some ion-exchangers and cleaning compresses applied; (b) ammonium-carbonate paste at the edge of the leading; (c) segment of glass after treatment; (d) panel sV 4d after treatment.
Evaluation and checking of these cleaning tests were carried out in the workshops during the course of designated meetings. In addition to visual evaluation of the improvements in transparency, the progress of which was also documented photographically, the effectiveness of the poultices was ascertained. Cleaning potential, defined as the level of calcium sulphate dissolved, was measured by ion-chromotography (fig. 34) (Jägers and Brinkmann 1999). Ion-exchanger poultices proved to have a much higher capacity for dissolving calcium sulphate. The cleaning effect of the first application was astonishingly effective, while subsequent applications only removed very low levels of sulphate. It is therefore recommended not to have more than two or three applications.
-

- Fig. 34. Measurement of the amount of calcium sulphate dissolved by cleaning compresses: results of the ion-chromatography, with the concentrations of sulphate given in mg per g of dried cleaning compress). Formulas: AC (10% ammonium-carbonate solution applied with Tylose C300 and Arbocel) and IT (strongly basic ion-exchanger equipped with carbonate, applied with Tylose C300 and 10% ammonium-carbonate solution). Samples: R (red glass from nVII 3c), B (dark-blue glass from sV 4d), and V (violet glass from sV 4d).
Parallel to the cleaning tests on the corrosion layers, infra-red-spectroscopic analysis showed that the transformation of sulphate ions into carbonate ions occurred extremely quickly; indeed, the process was almost completed after the first application (Jägers and Brinkmann 1999).
Both cleaning methods, carbonate ion-exchangers and ammonium-carbonate poultices, achieved a similar cleaning effect on the original glass. The poultices have the advantages of being easy to prepare and use, though in all probability complete removal of dissolved-sulphate residues from the surface is only possible with ion-exchangers. After treatment cycles totalling 65 to 88 hours, improvements in transparency were observed in several segments of glass from panels sV 4d and nVII 3c; these were judged to be sufficient to improve the readability of the glass. Undissolved remains left on the glass after cleaning, giving the surface a slightly flecked appearance, were identified as highly calcitic material from the corrosion-product encrustation. Attempts to remove these with a nitrate-coated anion-exchanger were not successful. Infra-red tests on model glass showed that all solvent residues could be removed simply by careful washing after treatment with a sponge and distilled water: such residues would have been a cause for concern that undesired reactions might occur subsequently.
5. Conclusions
Within the context of this research programme, scientists and conservators collaborated to assess various cleaning methods for historical stained glass. For cleaning glass surfaces there are the mechanical methods already being applied in most workshops, as well as the new chemical cleaning possibilities. Laboratory experiments were conducted initially on model glass in order to test the effectiveness and damage potential of these various methods; the model glass was examined both before and after cleaning by means of light microscopy and infra-red spectroscopy. In addition to this, cleaning tests were carried out on samples of original glass; electron-ray micro-analysis was also used to evaluate the results of these tests. The model-glass samples were corroded in advance by accelerated weathering in an attempt to simulate the damage seen on medieval glass. Enough model glass was prepared to allow any number of series of experiments to be carried out, in order to compare the effectiveness and suitability of the various chemical and mechanical methods and techniques.
For the mechanical cleaning tests, a wide range of selected tools was tested by different conservators on pre-corroded model glass. This showed clearly that the success (or lack of it) of a cleaning episode was determined not just the choice of tool, but by the skill with which conservator wielded it. This observation was later confirmed by research on samples of original glass. Insensitive handling of tools or unsuitable tools could damage or completely destroy the gel layer. The gel layer should not be damaged, as this leads to increased levels of corrosion subsequently, though the encrustations should be reduced, as their hygroscopic qualities accelerate the corrosion processes.
At the next stage, chemical cleaning methods were tested, and the results of cleaning with organic solvents (toluene, ethanol, acetone, etc.) and water-based solutions (oxalic acids, EDTA, ammoniac, etc.) compared with one another and evaluated. It became clear that strongly acidic and alkaline solutions, depending on the condition of the surface, could cause damage or have uncontrollable consequences.
In the specific case of the glass from Erfurt Cathedral, for whose firmly fixed, very hard, opaque encrustations mechanical methods proved to be either ineffective or damaging to the glass, new chemical cleaning possibilities had to be found. Following comprehensive preliminary tests on model glass, various types of ion-exchanger and ammonium-carbonate solution were chosen. Both methods were successful in improving the transparency of some (but not all) of the samples.
The experiments with the original material from Erfurt Cathedral clearly showed that successful cleaning was dependent on the particular composition of the encrustation. Really gentle cleaning can be achieved by chemical means, though only when the chemical agents are tailored to individual damage situations; this can only be achieved when scientific analysis is undertaken in tandem with the cleaning processes.
The assessment of these mechanical and chemical cleaning methods was oriented towards treating corrosion on sensitive medieval glass. Nineteenth-century glass is generally less sensitive, though cleaning of this material must also be careful and very gentle. Scientific analysis alone is not enough to determine the choice of cleaning method, but can act as an aid to the conservator in selecting the right cleaning agent and method.
6. Acknowledgements
Authors: Hannelore Römich, Elisabeth Jägers, Manfred Torge, Wolfgang Müller, Karin Adam. This text originally appeared as chapter 5, 'Reinigung - eine Gratwanderung' (pp. 101–28), in A. Wolff (ed.), Restaurierung und Konservierung historischer Glasmalereien, Mainz, 2000.
Publisher: Verlag Philipp von Zabern.
Translators: Joseph Elders, Joseph Spooner, Sebastian Strobl.
Images: Dombauhütte Köln, Glaswerkstatt (fig. 1); E. Jägers, Fachhochschule Köln (figs 2, 3, 34); Fraunhofer-Institut für Silicatforschung (ISC), Würzburg, Außenstelle Bronnbach (figs 4–9); Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin (figs 10–32); Dombauamt Erfurt, Glaswerkstatt (fig. 33).